MATERIALS & METHODS
All chemicals were purchased from Wako Pure Chemicals
(Osaka, Japan), unless otherwise stated.
Centrifugation of liposuction aspirates
Liposuction aspirates were obtained from surgery
performed on the abdomen or thigh regions of 8 healthy
female donors aged 21?38 y.o with informed consent
which was approved by our institutional review board.
Infiltration of saline (tumescent solution) and
liposuction and subsequent centrifugation of syringes
were conducted using a single combined machine (LipokitR,
Medikan Corp., Seoul, Korea) (Fig. 1A). Liposuction
aspirates, which consist of the fatty and fluid
portions, were divided and poured into disposable
sterilized syringes (50 ml) with a filter piston
(Medikan Corp., Seoul, Korea). The filter piston
was specifically designed for separation of the
oil from the other portions by centrifugation (Fig.
1B). After being placed upright for 10 minutes,
specimens were optically divided into two portions:
a floating adipose portion and a denser fluid portion.
Syringes were allotted to 6 groups (two syringes
each), and centrifuged at 0, 400, 800, 1200, 3000,
or 4200 ×g by LipokitR for 3 minutes. After centrifugation,
specimens were optically divided into three portions;
oil (top), adipose (middle), and fluid (bottom)
(Fig. 1B, C). The volume and weight of each portion
were measured before and after centrifugation, and
the specific gravity (= weight (g)/volume (ml))
of the adipose portion was calculated.
Counting of blood cells and adipose-derived
cells
Although blood cells are thought to disturb engraftment
of injected adipose and to be partly extracted by
centrifugation, there are no reliable data on the
extent of blood cell clearance by centrifugation.
Adipose portion tissues before and after centrifugation
were digested at 37oC for 30 min on a shaker with
an equal volume of 0.075% collagenase. Mature adipocytes
and connective tissues were separated from pellets
by centrifugation (400 ×g, 10 min). The pellet was
resuspended in PBS and passed through a 100-μm mesh
filter (Millipore, MA, USA). Then numbers of red
blood cells (RBCs) and nucleated cells were counted
with CellTec (Nihon Koden, Tokyo, Japan). Fluid
portions (3 ml each) before and after centrifugation
were centrifuged (400 ×g, 10 min), and the pellets
were resuspended and passed through a 100-μm mesh
filter. Numbers of RBCs and nucleated cells were
also counted. Nucleated cells counted corresponded
to WBCs, ASCs, and other adipose-derived cells such
as endothelial cells.
Isolation, culture, and counting
of ASCs from the adipose and fluid portions
ASCs were separately isolated from the adipose and
fluid portions of liposuction aspirates. Briefly,
fatty portions were digested with an equal volume
of 0.075% collagenase in PBS for 30 min on a shaker
at 37oC. Mature adipocytes and connective tissues
were separated from pellets by centrifugation (400
×g, 10 min). Pellets were resuspended, passed through
a 100-μm mesh filter, and washed with PBS. Fluid
portions were centrifuged (400 ×g, 10 min), and
the pellets were resuspended in PBS and passed through
a 100-μm mesh filter. After centrifugation (400
×g, 10 min), pellets were resuspended and washed
with PBS. Freshly isolated cells from the fatty
and fluid portions were plated in medium on 100-mm
gelatin-coated dishes. The stromal vascular fraction
cells were cultured with M-199 medium containing
10% FBS, 100 IU penicillin, 100 mg/ml streptomycin,
5 ng/ml heparin, and 2 ng/ml acidic fibroblast growth
factor, at 37oC, 5% CO2, in humid air. The cells
were cultured for seven days and cell counts were
performed using a NucleoCounter (Chemometec, Denmark).
Fat transplantation to nude mice
To examine the influences of centrifugation on engraftment
of adipose tissue, 5-week-old nude mice housed with
free access to water and standard chow diet were
used as recipients of human fat transplantation.
One milliliter of uncentrifuged or centrifuged adipose
tissue was subcutaneously injected into the back
by using an 18-gauge needle. Animals were sacrificed
4 weeks after fat transplantation, and transplanted
grafts were dissected and measured for weight. Harvested
samples were fixed and processed for histology (see
below). To estimate the net efficacy of transplantation
per volume of adipose portion before centrifugation,
a calculation was performed with the hypothetical
equation:
putative graft take of 1 ml uncentrifuged
adipose =
(graft take of 1 ml centrifuged adipose) (volume
of adipose portion after centrifugation)/(volume
of adipose portion before centrifugation).
Scanning electron microscopic study
Scanning electron microscopic (SEM) observation
was performed on samples after settlement and centrifugation
and on transplanted adipose obtained 4 weeks after
transplantation. Adipose samples were fixed with
paraformaldehyde and 2.5% glutaraldehyde in 0.2
M cacodylate buffer for a week at room temperature,
and then fixed in 1% osmium tetroxide. After dehydration,
they were dried with a supercritical point CO2 dryer
(HCP-2, Hitachi, Tokyo, Japan), sputter-coated with
Pt-Pd, and examined with a scanning electron microscope
(S3500N, Hitachi). Light microscopic examination
of hematoxylin-and-eosin?stained slides were also
performed
Statistical analyses
Results were expressed as mean ± standard error
of mean. Paired t-tests were performed to evaluate
the differences between centrifugal conditions.
Statistical significance was defined as p < 0.05.
RESULTS
Gross effects of centrifugation on adipose portion
of liposuction aspirates
With increased centrifugal force, the volumes of
the oil and fluid portions increased, and the volume
of the adipose portion decreased. Although there
was no significant difference between 3000 ×g and
4200 ×g, volumes of oil, adipose, and fluids altered
significantly with increased centrifugal forces
(Fig. 2A). The specific gravity of the adipose portion
did not change significantly except for a decreased
change between control and 400 ×g (Fig. 2B).
On SEM observation, the adipose portion was observed
as clusters of spherically shaped adipose cells.
Adipocyte size was not remarkably altered with increased
centrifugal forces. In all samples, including uncentrifuged
controls, clusters of adipocytes were partially
ruptured. The degree of ruptured adipocyte clusters
seemed to vary among donor subjects, but no remarkable
difference was seen between the samples processed
with different centrifugal forces from the same
subject (Fig. 3).
Effects of centrifugation on numbers
of RBCs, ASCs, and nucleated cells
The numbers of RBCs, ASCs, and nucleated cells in
the adipose and fluid portions were separately counted
before and after centrifugation. RBCs and nucleated
cells were counted as freshly isolated cells, while
ASCs were counted as cultured adherent cells after
1 week of cell culture. The cell numbers in the
adipose and fluid portions were compared to assess
distributional changes by centrifugation from the
adipose portion to the fluid portion or vice-versa.
Although the total number of RBCs in the adipose
and fluid portions did not change significantly
based on centrifugal forces (Fig. 4A), RBCs significantly
shifted from the adipose portion to the fluid portion
at all different centrifugal forces compared to
control. In addition, a significant difference was
seen between 400 ×g and 700 ×g but not between 700
×g and more than 1200 ×g (Fig. 5A). Total numbers
of nucleated cells, which included WBCs, ASCs, and
other adipose-derived cells, did not significantly
change by centrifugation; neither were there statistically
significant shifts in numbers of nucleated cells
detected for any centrifugal force (data not shown).
The total number of ASCs remained consistent up
to 1200 ×g, while the number of ASCs significantly
decreased at more than 3000 ×g (Fig. 4B). Unlike
RBCs, ASCs did not shift significantly between the
adipose and fluid portions by centrifugation (Fig.
5B).
Adipose graft survival of in vivo
experimental models
Four weeks after transplantation, weights of adipose
grafts, which were originally 1 ml of uncentrifuged
or centrifuged aspirated adipose, were measured.
It was revealed that centrifugation significantly
enhanced the proportion of graft survival (Fig.
6A). Significance was detected not only between
control and all centrifugal forces, but also between
increased centrifugal forces, although results from
centrifugation at 3000 ×g were superior to those
from 4200 ×g centrifugation (Fig. 6A).
Centrifugation made the original volume of fat compact;
for example, 1 ml fat centrifuged at 3000 ×g was
originally 1.55 ml before centrifugation. Thus,
uncentrifuged 1 ml fat and centrifuged 1 ml fat
differed originally in fat volume. If we have a
sufficient volume of aspirated fat, we can conclude
that centrifugation can enhance the graft take.
On the other hand, if we tried to obtain the largest
adipose graft by using 1 ml of uncentrifuged adipose
alone, it could be concluded by virtual calculation
that the uncentrifuged graft would be better than
any centrifuged grafts; centrifugation would not
contribute to enhancement of final graft take (Fig.
6B).
Under microscopic observation, no remarkable difference
was observed in cell integrity or structure among
samples centrifuged at different centrifugal forces.
Even in samples centrifuged with a maximum force
of 4200 ×g, adipocytes survived well 4 weeks after
transplantation (Fig. 6C).
DISCUSSION
Concentration of the graft
Although various authors have recommended performing
pre-centrifugation of fat grafts, many reports described
centrifugal force using rpm (rate per minute) units.1,4,6,8,11,14,24-27
Working centrifugal forces with the same rpm value
can differ in terms of radius of centrifugation,
meaning that they differ based on the individual
centrifugation device. Thus, it is hard to compare
our results with those from other previous reports,
especially previous reports that used rpm.
Our study showed that 3 minutes of centrifugation
compacts the adipose portion of liposuction aspirates
and partly excludes oil, water, and blood cells,
but not ASCs from the aspirated adipose. Consequently,
adipose tissue, extracellular matrix, and ASCs are
concentrated by centrifugation, likely contributing
to a boost in the graft take.28,29 The degrees of
concentration and exclusion tended to be elevated
with increased centrifugal force.
Damage to adipocytes
Boschert et al. reported that the quantity of oil
increased when specimens were centrifuged at more
than 100 ×g, and they concluded that the increase
in oil resulted from adipocyte destruction and that
centrifugation at greater than 100 ×g was not appropriate
for autologous fat transplantation.6 However, the
results of our fat graft experiments indicate that
centrifugation with more than 100 ×g centrifugal
force can surely be used in fat transplantation.
Our results showed that the volume of the oil portion
increased with increased centrifugal forces, but
histological findings did not clearly suggest destruction
of adipocytes. Based on our microscopic observations,
morphologically broken adipocytes were observed
even in uncentrifuged samples. The degree of adipocyte
destruction differed among patients but showed only
minor differences between different centrifugal
forces. SEM observation showed that remnant oil
was seen in the adipose portion even after 3 minutes
of centrifugation. Thus, we suggest that the increase
in the oil portion does not necessarily mean an
increase adipocyte destruction by centrifugation
but may rather mean an increase in separation of
oil from the adipose portion.
Damage and distribution of blood
cells and ASCs
Our results are mostly in accordance with the view
previously reported that centrifugation separates
fat cells from lipid, blood cells, water, and water-soluble
ingredients such as proteases and lipases.4,7-10
To our knowledge, there have been no reports examining
quantitatively the effects of centrifugation on
blood cells and ASCs in liposuction aspirates. Our
results showed that total numbers of RBCs did not
significantly change by centrifugation and that
RBCs partly changed their location from the adipose
portion to the fluid portion by centrifugation.
However, the volume of the adipose portion was compacted
to a greater extent than the shift of blood cells,
and thus these blood cells were slightly concentrated
in the adipose portion. Although a previous author
indicated that the presence of blood in the region
of the injected fat stimulates macrophage activity
to remove the fat cells,8 the actual effect of the
blood in the graft has not clearly been elucidated.
Thus far, we cannot determine whether a decrease
of number and increase of concentration of RBCs
and WBCs is advantageous or disadvantageous in fat
grafting.
On the other hand, the results indicated that ASC
yield after 1 week of culture was almost consistent
up to 3000 ×g and decreased at more than 3000 ×g. In
addition, it was shown that ASCs did not shift between
the adipose and fluid portions by centrifugation,
likely because ASCs contained in the adipose portion
are resident in or strongly adhered to the adipose
tissues. Accordingly, centrifugation simply enhanced
the density of ASCs in the fat graft as a result
of compaction of the adipose portion. Condensation
of ASCs in the graft may be beneficial for enhancing
the fat graft survival rate for the reasons discussed
below.
Graft survival
We suggest that aspirated fat graft takes were grossly
influenced by the balance of the negative effects
of destruction and positive effects of condensation
by centrifugation. Our results revealed that the
short-term survival rate of aspirated adipose graft
per volumetric unit after centrifugation increased
with centrifugal forces up to 3000 ×g. Condensation
of the graft material as well as ASCs is thought
to dominantly contribute to this enhancement.
However, it was also suggested by a virtual calculation
that surviving fat graft per volumetric unit before
centrifugation decreased by intervention of centrifugation.
The decrease of surviving fat graft occurred at
400 ×g and was not progressively enhanced with further
increased centrifugal forces. This decrease at 400
×g may result from the destruction of adipocytes
located especially in the superficial layers of
adipose fragments.
Histological findings of transplanted adipose tissues
were consistent with previous reports,8,30 which
found that centrifuged graft samples were similar
to uncentrifuged ones. Even samples centrifuged
at 4200 ×g showed no remarkable differences in histology
from controls after transplantation. It is thus
suggested that once adipocytes succeed in avoiding
critical damage during centrifugation, there will
be no difference in the structural quality of adipocytes
between centrifuged and uncentrifuged samples.
Our results of graft takes with or without centrifugation
suggest a clinical implication for selective use
of centrifugation. If we had only a restricted amount
of adipose (though it would be very rare), it might
be better not to use centrifugation from the standpoint
of utmost effective use of restricted graft material.
However, in most clinical cases, it is easy to harvest
a sufficient volume of aspirated fat, and in such
cases, we should centrifuge aspirated fat before
grafting to obtain the best augmentation effects.
Excessive centrifugation can destroy adipocytes
and ASCs. Centrifugation, however, plays a beneficial
role in concentrating adipocytes, ECM, and ASCs,
and in partially excluding RBCs. ECM should maintain
its volume after transplantation at least in the
short term, and exclusion of RBCs from graft materials
may contribute to a better survival rate of transplanted
adipose. We suggest that ASCs and other adipose-derived
cells are crucial for graft survivability in both
the short- and long term. Our recent report28 revealed
that aspirated fat is relatively ASC deficient compared
to excised whole fat, which contains large vessels
and nerves, unlike aspirated fat, and that ASCs
can survive and reside between adipocytes or in
the connective tissues of surviving adipose tissue
after transplantation. Condensation of ASCs by centrifugation
may mean conversion of relatively stem-cell?deficient
adipose to relatively stem-cell?rich adipose. This
ASC condensation may enhance the fat graft take28
and may prevent long-term atrophy of transplanted
adipose by working as tissue-specific progenitors.28,29
RBCs were shifted at 400 ×g. ASCs were damaged at
3000 ×g. Graft takes of centrifuged adipose were
best at 3000 ×g. Taken together, these data lead
us to tentatively recommend 1200 ×g as an optimized
centrifugal force among all tested centrifugal forces
for obtaining short-term and long-term good results
in adipose transplantation. It is interesting that
our conclusion is similar to the 1286 ×g recommended
by Coleman based on abundant clinical experience.32
We, however, have to be cautious in interpreting
the experimental results because the gross take
of transplanted tissue will be clinically influenced
by various factors associated with procedures of
harvesting, processing, and transplanting of adipose
tissues.
FIGURE LEGENDS
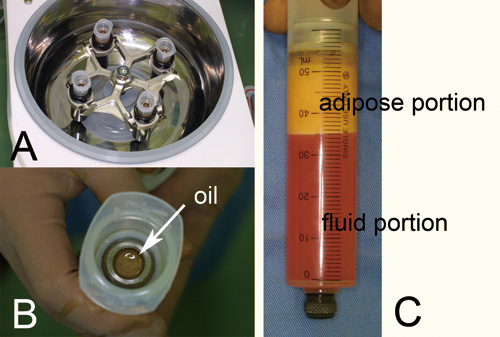
Figure 1. Centrifugation of liposuction aspirates.
(A) A machine for centrifugation used in this study
(LipokitR, Medikan Corp., Seoul, Korea). Infiltration
of tumescent solution and liposuction were also
performed with this combined machine.
(B) A disposable sterilized syringe (50 ml) with
a filter piston (Medikan Corp., Seoul, Korea). By
centrifugation, oil was shifted onto the piston.
(C) The adipose and fluid portions of liposuction
aspirates were clearly separated by 3 minutes of
centrifugation and stayed below the piston after
that.
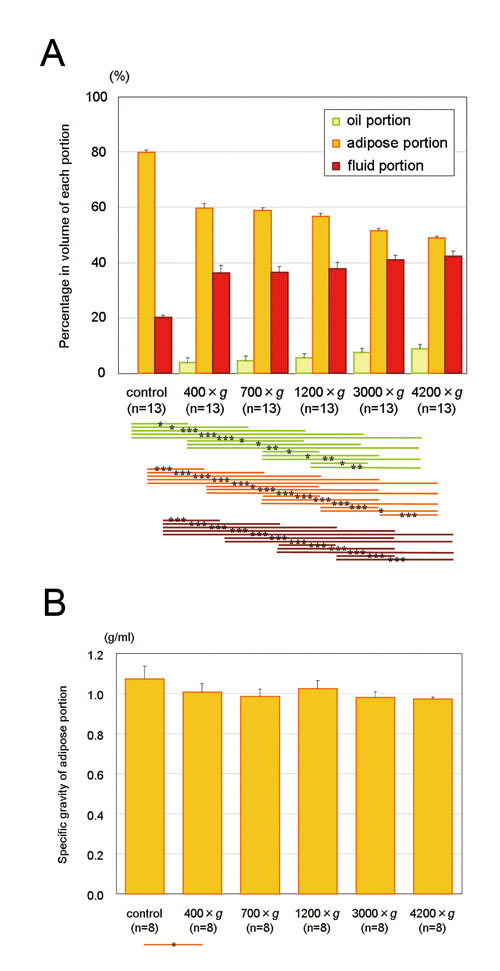
Figure 2. (A) The adipose portion was concentrated
to 74.7, 73.6, 71.0, 64.5, and 61.3% in volume after
3 minutes of centrifugation at 400, 700, 1200, 3000,
and 4200 ×g, respectively. A significant volume
reduction in the adipose portion was observed not
only between uncentrifuged and centrifuged samples
but also between different centrifugal forces. The
fluid portion and oil portion also significantly
increased in volume in a centrifugal force-dependent
manner. Statistical analysis was performed by paired
t-tests between groups. *: p< 0.05, **: p<
0.01, ***: p< 0.001. (B) Specific gravity of
the adipose portion tends to decrease with increased
centrifugal force, but the differences were not
statistically significant. Data represent means
± SEM.
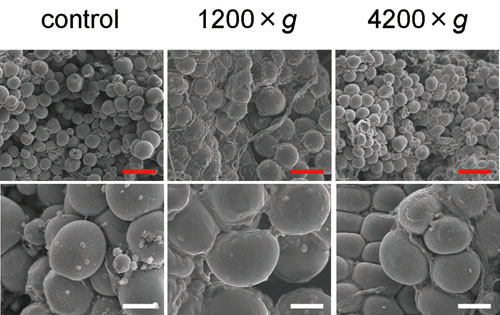
Figure 3. SEM photos of representative uncentrifuged
and centrifuged samples derived from a single donor.
Cluster of spherically shaped adipocytes and intermittently
dispersed ruptured cells were observed, regardless
of centrifugal forces (left: uncentrifuged; center:
1200 ×g; right: 4200 ×g). Magnified photos show
that there are adipocytes that were not morphologically
altered even in samples centrifuged at 4200 ×g (bottom
right). Scale bar = 200 μm (top) and 50 μm (bottom).
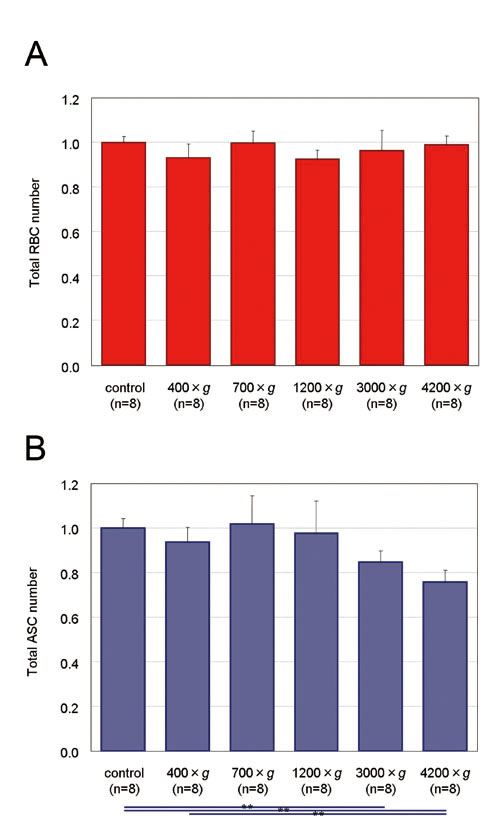
Figure 4. Total numbers of RBCs and ASCs in the
adipose and fluid portions of liposuction aspirates
before and after centrifugation. Uncentrifuged control
samples. Proportions of total count of each centrifugal
condition to control are presented. Significance
was analyzed using paired t-tests for groups. Data
represent means ± SEM. *: p< 0.05, **: p<
0.01, ***: p< 0.001.
(A) Total numbers of RBCs did not change significantly
by centrifugation.
(B) Total number of ASCs showed no remarkable alteration
after centrifugation up to 1200 ×g, but a significant
decrease was observed between controls and samples
centrifuged at 3000 and 4200 ×g.
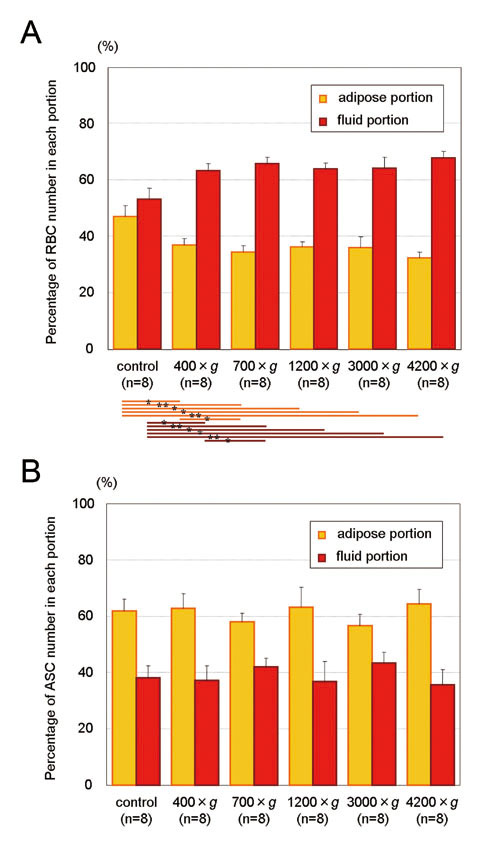
Figure 5. Shift of RBCs and ASCs between the adipose
and fluid portions by centrifugation. Proportions
of cell numbers contained in adipose and fluid portions
before and after centrifugation are presented. Significance
was analyzed using paired t-tests between groups.
Data represent means ± SEM. *: p< 0.05, **: p<
0.01, ***: p< 0.001.
(A) Percentages of RBC count contained in the adipose
and fluid portions. Number of RBCs in the adipose
portion decreased to 79.7, 77.8, 85.7, 86.4, and
73.7% of that of uncentrifuged control as a result
of 3 minutes centrifugation at 400, 700, 1200, 3000,
and 4200 ×g, respectively. RBCs significantly shifted
from the adipose portion to the fluid portion at
all different centrifugal forces compared to control.
In addition, significance was seen between 400 ×g
and 700 ×g, but not between 700 ×g and more than
1200 ×g, suggesting that centrifugation at 700 ×g
is enough and that more than 700 ×g may not be necessary
for RBC extraction from aspirated adipose.
(B) Percentage of ASC count contained in the adipose
and fluid portions. ASCs did not significantly shift
between the adipose and fluid portions at any level
of centrifugal force.
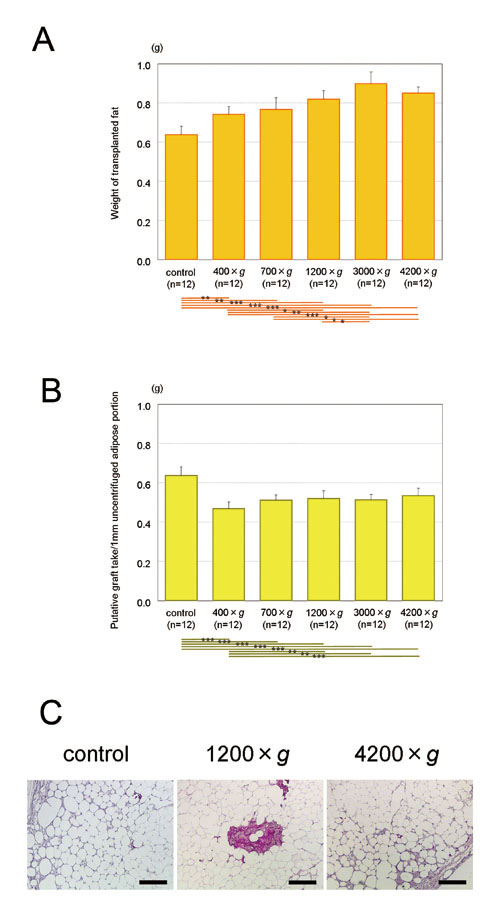
Figure 6. Transplantation of uncentrifuged and centrifuged
adipose tissue. Human aspirated adipose (1 ml) was
transplanted into the back skin of nude mice with
or without centrifugation, and the surviving adipose
tissues were harvested 4 weeks later. Significance
was analyzed with paired t-tests between groups.
Data represent means ± SEM. *: p< 0.05, **: p<
0.01, ***: p< 0.001.
(A) Weights of transplanted adipose tissues. With
centrifugation at 1200 ×g or more, transplanted
adipose tissue was significantly greater in weight
than uncentrifuged control. Centrifugation significantly
contributed to obtaining a better graft take at
least in short-term observations, although centrifugation
at 4200 ×g might be excessive compared to 3000 ×g.
(B) Calculated putative graft take per volume of
uncentrifuged adipose. Values were calculated as
follows. Putative graft take of 1 ml uncentrifuged
adipose = (graft take of 1 ml centrifuged adipose)
(volume of adipose portion after centrifugation)/(volume
of adipose portion before centrifugation). Based
on these putative calculations, if there was a limited
volume of aspirated adipose, graft take would be
best when it was not centrifuged before transplantation.
(C) Hematoxylin-Eosin findings of surviving adipose
tissues (left: uncentrifuged; middle: 1200 ×g; right:
at 4200 ×g). Spherically shaped, viable adipocytes
were observed regardless of centrifugal forces.
No differences were found between the samples centrifuged
with different forces. Scale bar = 200 μm.
REFERENCES
1. Coleman, S.R. The technique of periorbital lipoinfiltration
Operative Techniques in Plastic and Reconstructive
Surgery 1:120, 1994.
2. Niechajev, I., Sevcuk, O. Long-term results of
fat transplantation: clinical and histologic studies.
Plast. Reconstr. Surg. 94: 496, 1994.
3. Brandow, K., Newman, J. Facial multilayered micro
lipo-augmentation Int. Journal of Aestheric and
Restorative surgery, 1996; 4(2): 1996
4. Fulton, J.E., Suarez, M., Silverton, K., Barnes,
T. Small volume fat transfer. Dermatol. Surg. 24:
857, 1998.
5. Coleman, W.P. 3rd. The history of liposuction
and fat transplantation in America. Dermatol. Clin.
17: 723, 1999.
6. Sommer, B., Sattler, G. Current concepts of fat
graft survival: histology of aspirated adipose tissue
and review of the literature. Dermatol. Surg. 26:
1159, 2000.
7. Donofrio, L.M. Structural autologous lipoaugmentation:
a pan-facial technique. Dermatol. Surg. 26:1129,
2000.
8. Shiffman, M.A. Effect of various methods of fat
harvesting and reinjection The American Journal
of Cosmetic Surgery 17: 91, 2000.
9. Shiffman, M.A., Mirrafati, S. Fat transfer techniques:
the effect of harvest and transfer methods on adipocyte
viability and review of the literature. Dermatol.
Surg. 27: 819, 2001.
10. Boschert, M.T., Beckert, B.W., Puckett, C.L.,
et al. Analysis of lipocyte viability after liposuction.
Plast. Reconstr. Surg. 109: 761, 2002
11. Butterwick, K.J. Lipoaugmentation for aging
hands: a comparison of the longevity and aesthetic
results of centrifuged versus noncentrifuged fat.
Dermatol. Surg. 28: 987, 2002.
12. Rohrich, R.J., Sorokin, E.S., Brown, S.A. In
search of improved fat transfer viability: a quantitative
analysis of the role of centrifugation and harvest
site. Plast. Reconstr. Surg. 113: 391, 2004.
13. Shiffman, M.A. Principles of autologous fat
transplantation. In Autologous Fat Transplantation
(ed. Shiffman MA), Marcel Dekker, Inc., New York,
NY, 2001, pp. 5-22.
14. Chajchir, A., Benzaquen, I., Moretti, E. Comparative
experimental study of autologous adipose tissue
processed by different techniques. Aesthetic Plast.
Surg. 17: 113, 1993.
15. Coleman, S.R. Structural fat grafts: the ideal
filler? Clin. Plast. Surg. 28:111, 2001.
16. Beckert, B. W., Puckett, C. L., and Concannon,
M. J. Analysis of loposuction aspirate for cell
viability at various centrifugation levels. Presented
at the Midwestern Association of Plastic Surgeons
Annual Scientific Meeting, Chicago, IL, April 21,
2002, and at the American Society for Aesthetic
Plastic Surgery and Aesthetic Surgery and Education
Foundation Annual Meeting, Las Vegas, NV, April
29, 2002.
17. Smith, P., Adams, W,P., Jr., Lipschitz, A.H.,
et al. Autologous human fat grafting: effect of
harvesting and preparation techniques on adipocytes
graft survival. Plast. Reconstr. Surg.117: 1836,
2006.
18. Katz, A.J., Llull, R., Hedrick, M.H., et al.
Emerging approaches to the tissue engineering of
fat. Clin. Plast. Surg. Oct;26: 587, 1999.
19. Tholpady, S.S., Llull, R., Ogle, R.C., et al.
Adipose tissue: stem cells and beyond. Clin. Plast.
Surg. 33: 55, 2006.
20. Zuk, P.A., Zhu, M., Mizuno, H., et al. Multilineage
cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7: 211, 2001.
21. Yoshimura, K., Sigeura, T., Matsumoto, D., et
al. Characterization of Freshly Isolated and Cultured
Cells Derived from the Fatty and Fluid Portions
of Liposuction Aspirates. J. Cell Physiol. 208:
64, 2006.
22. Miranville, A., Heeschen, C., Sengenes, C.,
et al. Improvement of postnatal neovascularization
by human adipose tissue-derived stem cells. Circulation.
110: 349, 2004.
23. Rehman. J., Traktuev, D., Li, J., et al. Secretion
of angiogenic and antiapoptotic factors by human
adipose stromal cells. Circulation. 109: 1292, 2004.
24. Ullmann, Y., Hyams, M., Ramon, Y., et al. Enhancing
the survival of aspirated human fat injected into
nude mice. Plast. Reconstr. Surg. 101:1940, 1998.
25. Shoshani, O., Shupak, A., Ullmann. Y., et al.
The effect of hyperbaric oxygenation on the viability
of human fat injected into nude mice. Plast. Reconstr.
Surg. 106: 1390, 2000.
26. Shoshani, O., Ullmann. Y., Shupak. A., et al.
The role of frozen storage in preserving adipose
tissue obtained by suction-assisted lipectomy for
repeated fat injection procedures. Dermatol. Surg.
27: 645, 2001.
27. Ramon, Y., Shoshani, O., Peled, I.J., et al.
Enhancing the take of injected adipose tissue by
a simple method for concentrating fat cells. Plast.
Reconstr. Surg. 115: 197, 2005.
28. Matsumoto, D., Sato, K., Gonda, K., et al. Cell-assisted
lipotransfer (CAL): supportive use of human adipose-derived
cells for soft tissue augmentation with lipoinjection.
Tissue Eng., in press.
29. Masuda, T., Furue, M., Matsuda, T. Novel strategy
for soft tissue augmentation based on transplantation
of fragmented omentum and preadipocytes. Tissue
Eng. 10:1672, 2004.
30. Carpaneda, C.A., Ribeiro, M.T. Study of the
histologic alterations and viability of the adipose
graft in humans. Aesthetic Plast. Surg. 17: 43,
1993.

