|
Introduction
The combination of topical tretinoin (all-trans retinoic
acid; atRA) and hydroquinone is effective on various
hyperpigmented skin disorders. Kligman and Willis1
introduced this treatment, and several modified protocols2
have been reported. In these therapeutic regimens,
topical corticosteroids are used in combination with
atRA and hydroquinone in order to reduce adverse effects
such as irritation and erythema. We have previously
proposed aggressive bleaching protocols,3-8 in which
atRA and hydroquinone were used separately. Corticosteroids
were not used, because corticosteroids reduce the
melanin-discharging effect of atRA. Corticosteroids
can lead to postinflammatory hyperpigmentation in
bleaching therapy, especially in colored skin, probably
by suppressing epidermal turnover and melanin discharge.
Since 1995, we have successfully treated more than
15,000 cases of various skin lesions with epidermal
hyperpigmentation using our bleaching protocols.
In our combined bleaching treatment with atRA and
hydroquinone, atRA discharges melanin granules from
the epidermis by accelerating epidermal turnover,7,9
while hydroquinone strongly suppresses new melanin
production.10 The hyperpigmented epidermis is replaced
by less-pigmented epidermis in a few weeks. AtRA promotes
the discharge of melanin granules in epidermis by
(1) accelerating epidermal turnover (differentiation
of keratinocytes) in a direct manner, and (2) promoting
epidermal growth (proliferation of keratinocytes)
in an indirect manner; the latter effect was found
to be mediated by HB-EGF secreted by suprabasal keratinocytes.9,11
Thus, atRA has a specific effect as a discharger of
epidermal melanin that cannot be performed by any
other reagents or any peeling procedures such as alpha-hydroxyl
acid peeling or microdermabrasion. These exfoliating
procedures simply induce subsequent normal wound healing
and only minimal acceleration of epidermal turnover.
However, it is well known that atRA frequently induces
irritant dermatitis, especially when used aggressively.
As all-trans retinol (ROL) and all-trans retinal had
been considered to be less irritating than atRA,12,13
we tried using 10% ROL aqueous gel instead of 0.1%
atRA gel and achieved similar effectiveness, but failed
to reduce the adverse effects.6 As yet, there is no
way to reduce the irritant dermatitis without losing
the bleaching efficacy of retinoids.
Nanoscale atRA (nano-atRA) particles were developed
as a novel drug delivery system through the use of
a boundary-organized nanoscale reaction.14 The poor
stability of atRA to heat and light is a serious pharmacological
problem: our hand-made, aqueous atRA gel requires
monthly preparation.3-8 Nano-atRA showed improved
stability compared with atRA,14 and can be stocked
for 6 months. In addition, nano-atRA particles showed
improved permeation of the stratum corneum, and atRA
should be gradually released in the epidermis as nanoscale
micells degrades. In murine skin, nano-atRA gel contributed
to accelerated epidermal turnover, epidermal hyperplasia,
decreasing melanin content, increased expression of
hyaluronic acid, and increased heparin-binding epidermal
growth factor-like growth factor (HB-EGF) mRNA levels
in epidermal keratinocytes.14 Enhanced production
of HB-EGF by suprabasal keratinocytes is known to
be one of the important phenomena seen after topical
retinoid treatment11 and is the most likely mechanism
by which retinoids accelerate the discharge of epidermal
melanins.9
Improved permeation and slow release of atRA from
nanoscale particles may enhance its clinically beneficial
effects and/or reduce its adverse effects, such as
retinoid dermatitis. In this trial study, we prepared
0.1%, 0.2%, and 0.4% nano-atRA gels for clinical use
in order to estimate the bleaching potential of nano-atRA
gel and the extent of its adverse side effects.
Methods
Preparation of Ointments
AtRA was commercially obtained from Wako Pure Chemical
Industries Ltd. (Osaka, Japan), and 0.1, 0.2, and
0.4% nano-atRA gels were prepared using boundary-organized
nanoscale reaction droplets as previously reported14
(Figure 1). The size and structure of the nano-atRA
particles were confirmed by freeze fracture transmittance
electron microscopy (ff-TEM, Hitachi Co. Ltd.). The
nano-atRA gels were prepared at Institute of Medical
Science, St. Marianna University School of Medicine.
An ointment including 5% hydroquinone and 7% lactic
acid (HQ-LA ointment) and an ointment including 5%
hydroquinone and 7% ascorbic acid (HQ-AA ointment)
were also prepared. Plastibase (petrolatum polyethylene
ointment base; Taisho Pharmacology, Osaka, Japan)
was used as the ointment base of the HQ-LA ointment,
whereas a hydrophilic ointment (Taisho Pharmacology,
Osaka, Japan) was used for the HQ-AA ointment.
Patients
Each ointment was applied topically by 83 Japanese
women and 1 man with facial hyperpigmented skin lesions,
and the 77 patients (76 women and 1 man) who were
followed up for more than 10 weeks were analyzed in
this study. As 11 patients had two lesions, there
were 88 hyperpigmented skin lesions in all, including
solar lentigines (n = 10), melasma (n = 36), ephelides
(n = 9), and post-inflammatory hyperpigmentation (PIH)
(n = 33). Dermal melanosis and melanocytosis were
not targeted. The other seven patients stopped the
treatment because of their poor compliance. The age
of the patients ranged from 16 to 84 years old (45.2
? 15.4; mean ? SD).
Treatment Protocol
Our bleaching protocol consists of two phases, a bleaching
phase and a healing phase.6-8 In the bleaching phase,
discharge of epidermal melanin is accelerated by nano-atRA
gel and melanin production is suppressed by HQ-LA
ointment. In the healing phase, the discharge of epidermal
melanin is discontinued and great care is taken not
to induce new post-inflammatory hyperpigmentation,
by using hydroquinone alone.
In the bleaching phase, 0.1% nano-atRA gel and HQ-LA
ointment were applied twice a day. Nano-atRA gel was
carefully applied only to pigmented areas, using a
small cotton-tip applicator, and subsequently HQ-LA
ointment was widely applied with the fingers beyond
the pigmented area (i.e., all over the face). Patients
were asked to visit our hospital at 1, 2, 4, 8, and
12 weeks after starting this treatment. In most cases,
it took 2 to 8 weeks to finish this phase. When scaling
was not observed at 1 week, the concentration of tretinoin
was increased to 0.2% or 0.4%. The concentration of
tretinoin and frequency of its application were appropriately
modified according to the skin condition and the degree
of skin reaction.
The healing phase was begun after pigmentation had
improved sufficiently or 8 weeks had passed. The application
of nano-atRA gel and HQ-LA ointment was discontinued,
and application of HQ-AA ointment all over the face
was started. HQ-AA ointment was used until the erythema
was almost eliminated; it took 4 to 8 weeks to complete
this phase. Topical corticosteroids were not employed
in the bleaching or healing phase. In melasma patients,
a second treatment was performed after a month’s interval
if the patient requested it.
Evaluation of Results
Photographs of each patient were taken at baseline,
during, and after treatment with a high-resolution
digital camera (Nikon D70, Tokyo, Japan). The percentage
of pigmentary clearance was evaluated via the photographs
by two experienced plastic surgeons who did not perform
this treatment. The mean data of the pigmentary clearance
of each patient were classified into four categories:
excellent (80% clearance or better), good (50% to
less than 80% clearance), fair (0% to less than 50%
clearance), and poor (no change or worse).
Results
The 88 hyperpigmented lesions were treated for an
average period of 14.3 weeks. Almost all patients
had sufficient improvement without serious adverse
effects. Unpleasantness such as severe irritation
and erythema during treatment seemed to be seen less
frequently than in our thousands of cases treated
with conventional atRA gel3-8. Mild erythema appeared
in the first week in most cases and scaling was seen
at the same time. Nano-atRA gel appeared to induce
a lesser degree of erythema and a similar degree of
scaling in the first 2 weeks compared to conventional
atRA gel, although statistical analysis was not performed.
Scaling was observed even in the initial stage of
the healing phase, suggesting that the slow-release
nano-atRA gel had long-term effects. Resistance to
atRA, which always accompanies the conventional atRA
treatment, was also seen with the nano-atRA treatment.
The results of this study are summarized in Table
1. Forty-seven lesions (53.5%) were evaluated as ‘‘excellent’’,
22 (25.0%) as ‘‘good’’, 15 (17.0%) as ‘‘fair’’, and
4 (4.5%) as ‘‘poor’’. Most of the fair and poor cases
had minimal skin reactions, such as scaling, during
the bleaching phase. Representative cases are shown
in Figures 2-4.
Nano-atRA improved all four kinds of skin pigmentation
disorders. Of 36 cases of melasma, 28 (79.3%) ranked
“excellent” or “good”. The average treatment period
of melasma patients was 14.1 weeks. Thirty-two of
33 PIH cases ranked “excellent” or “good”. There were
no “poor” results in the PIH group. There were only
nine cases of ephelides, more than half of which achieved
“excellent” or “good” results. Only 4 of 88 lesions
(two of melasma and two senile lentigines) showed
“poor” treatment results.
Discussion
In our recent report, we used histology to confirm
that accumulated melanin granules around the basal
layer were cleared after treatment with atRA and hydroquinone.
In acquired dermal melanocytosis, melanin deposits
in the dermis appeared not to change.7 Thus, the bleaching
therapy is only effective for epidermal pigmentation,
and we therefore focused on various types of hyperpigmentation
of the epidermis in this trial.
Using nano-atRA gel based on the newly developed drug
delivery system technology, we obtained results as
good as those of previous studies that used our former
atRA gel. The present treatment resulted in satisfactory
improvement of melasma, with an average treatment
period of 14.1 weeks. Roughly two-thirds of the melasma
patients required only one session of the bleaching
treatment, while treatment of melasma with our former
atRA gel was sometimes performed in two or three sessions8.
Of the various hyperpigmented lesions treated, PIH
was most improved. This has always been true in our
treatment of lesions with conventional atRA. The number
of ephelides was small, but the majority of patients
had “excellent” or “good” results. Among the four
types of hyperpigmented lesions studied, the percentage
of excellent and good results was lowest in the senile
lentigines. Senile lentigines, especially those that
are resident for a long period, frequently show excessive
development of horny layers (hyperkeratosis) that
may obstruct the penetration of atRA into the epidermis.
This may also be the case with nano-atRA gel, though
high permeation of nano-atRA was reported in murine
skin.14 We recommend that irradiation by Q-switched
ruby laser (or other lasers) for removal of hyperkeratinization
of senile lentigines is first performed as done in
Case 1 (Figure 2). In colored skin, PIH after laser
treatments can frequently be a disfiguring problem.
However, PIH after laser irradiation is always easily
treated by the bleaching treatment described above.
In our conventional bleaching treatment, there are
two major problems: one is dermatitis induced by aggressive
use of atRA, and the other is the biochemical instability
of atRA in our ointments. We applied atRA only on
the pigmented area but used it aggressively, which
almost always led to irritant dermatitis on the area
where it was applied. The dermatitis is the most serious
side effect and remains to be resolved. In animal
experiments, nano-atRA gel showed slower diffusion
into the epidermis and higher degrees of epidermal
alterations, as assessed by retinoid signals such
as elevated HB-EGF mRNA expression, enhanced hyaluronic
acid deposition, and epidermal hyperplasia, with a
lesser degree of erythema.14 These advantages of nano-atRA
appeared to be partly confirmed in this clinical trial,
in which similar degrees of bleaching effectiveness
and scaling were seen, with a lesser degree of erythema.
In addition, the improved biochemical stability of
nano-atRA to heat and light is another advantage,14
as we had to prepare the conventional atRA gel every
month because of its pharmacological instability.
The fact that nano-atRA gel can be stored for at least
6 months without significantly losing atRA chemical
activity may increase its commercial profitability.
AtRA has long been used for treating acne, photoaging-associated
symptoms such as fine wrinkles, and hyperpigmentation.
When used aggressively, atRA can be an extremely powerful
tool for discharging epidermal melanin. Reducing retinoid-induced
dermatitis remains a major challenge. New generations
of synthetic retinoids or new drug delivery systems,
such as the nano-atRA presented here, may resolve
the problem.
Acknowledgement
Nano-atRA gels used in this study were generous gifts
from Dr. Yoko Yamaguchi and Dr. Rie Igarashi of Institute
of Medical Science, St. Marianna University School
of Medicine.
Table 1.
Results of the Nano-atRA Treatment
Protocol.
Average treatment period (weeks) Number of lesions
Excellent Good Fair Poor Total
Solar lentigines 13.6 1 3 4 2 10
Melasma 14.1 17 11 6 2 36
Ephelides 16.5 2 3 4 0 9
PIH 14.2 27 5 1 0 33
Total 14.3 47 22 15 4 88
PIH: postinflammatory hyperpigmentation
Table 2. Clinical Results of Cases with Melasma.
Number of cases
No. of treatments Excellent Good Fair Poor Total
Excellent Cases (%)
One treatment 12 10 5 1 28 42.8
Two treatments 5 1 1 1 8 62.5
Total 17 11 6 2 36 47.2
Figure legends
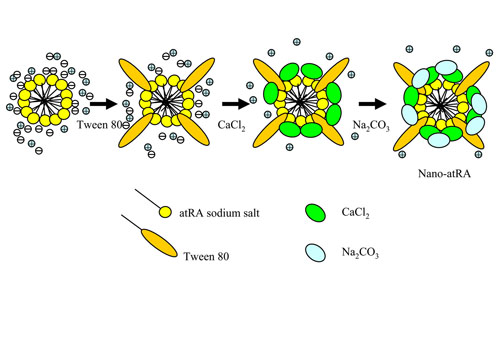
Figure 1. Schematic of the preparation of nano-atRA
particles with their core/shell (CaCO3) structure.
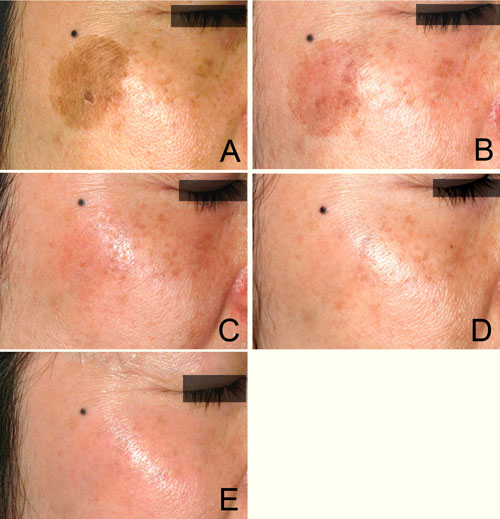
Figure 2. Case 1. A 40-year-old woman with a solar
lentigine and melasma on her right cheek, first shown
before treatment (A). The solar lentigine was first
treated with a Q-switched ruby laser, but 4 weeks
later PIH appeared at the original position (B). Bleaching
treatment with 0.1% nano-atRA gel and HQ-LA ointment
was performed for 4 weeks; the patient is shown at
2 weeks (C) and at 4 weeks (D). This was followed
by healing treatment with HQ-AA ointment alone for
4 weeks. Only minimal erythema appeared during the
bleaching phase. PIH was completely eliminated and
the melasma was also apparently improved at 8 weeks
(E).
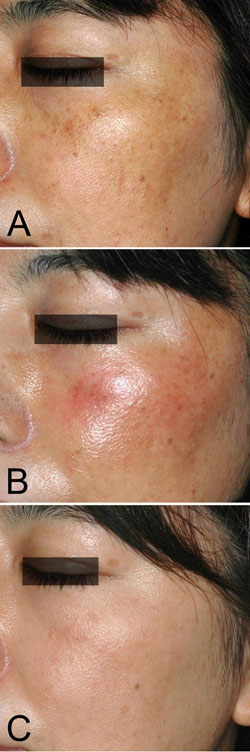
Figure 3. Case 2. A 38-year-old woman with melasma
on both cheeks is shown before the treatment (A).
Bleaching with 0.1% nano-atRA gel and HQ-LA ointment
was performed for 12 weeks, followed by application
of HQ-AA ointment alone for 4 weeks. Mild erythema
appeared during the bleaching phase, shown at 4 weeks
(B). The melasma was almost cleared at 16 weeks (C).
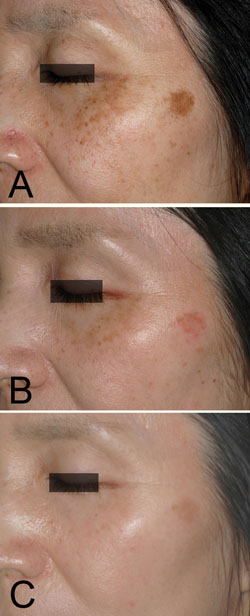
Figure 4. Case 3. A 53-year-old woman with a solar
lentigine and melasma on her left cheek before the
treatment (A). 0.1% nano-atRA gel was used together
with HQ-LA ointment in bleaching phase for 12 weeks,
and HQ-AA ointment was applied alone in the healing
phase for 9 weeks. Mild erythema was seen during the
bleaching phase, shown at 4 weeks (B). The melasma
almost disappeared, while the solar lentigine became
lighter, as seen at 21 weeks (C).
Reference
1) Kligman AM, Willis I. A new formula for depigmenting
human skin. Arch Dermatol 1875; 111: 40-8.
2) Gano SE, Garcia RL. Topical tretinoin, hydroquinone,
and betamethasone valerate in the therapy of melasma.
Cutis 1979; 23: 239-41.
3) Yoshimura K, Harii K, Aoyama T, et al. A new bleaching
protocol for hyperpigmented skin lesions with a high
concentration of all-trans retinoic acid aqueous gel.
Aesthetic Plast Surg 1999; 23: 285-91.
4) Yoshimura K, Harii K, Aoyama T, Iga T. Experience
with a strong bleaching treatment for skin hyperpigmentation
in Orientals. Plast Reconstr Surg 2000; 105: 1097-108.
5) Yoshimura K, Harii K, Masuda Y, et al. Usefulness
of a narrow-band reflectance spectrophotometer in
evaluating effects of depigmenting treatment. Aesthetic
Plast Surg 2001; 25:129-33.
6) Yoshimura K, Momosawa A, Aiba E, et al. Clinical
trial of bleaching treatment with 10% all-trans retinol
gel. Dermatol Surg 2003; 29: 155-60.
7) Momosawa A, Yoshimura K, Uchida G, et al. Combined
therapy using Q-switched ruby laser and bleaching
treatment with tretinoin and hydroquinone for acquired
dermal melanocytosis. Dermatol Surg 2003; 29: 1001-7.
8) Yoshimura K, Sato K, Aiba-Kojima E, et al. Repeated
treatment protocols for Melasma and Acquired Dermal
Melanocytosis. Dermatol Surg 2006; 32: 365-71.
9) Yoshimura K, Uchida G, Okazaki M, et al. Differential
expression of heparin-binding EGF-like growth factor
(HB-EGF) mRNA in normal human keratinocytes induced
by a variety of natural and synthetic retinoids. Exp
Dermatol 2003; 12(S2): 28-34.
10) Yoshimura K, Tsukamoto K, Okazaki M, et al. Effects
of all-trans retinoic acid on melanogenesis in pigmented
skin equivalents and monolayer culture of melanocytes.
J Dermatol Sci. 2001; 27(S1): S68-75.
11) Xiao J H, Feng X, Di W, et al. Identification
of heparin-binding EGF-like growth factor as a target
in intercellular regulation of epidermal basal cell
growth by suprabasal retinoic acid receptors. EMBO
J 1999; 18:1539-48.
12) Kang S, Duell EA, Fisher GJ, et al. Application
of retinol to human skin in vivo induces epidermal
hyperplasia and cellular retinoid binding proteins
characteristic of retinoic acid but without measurable
retinoic acid levels or irritation. J Invest Dermatol
1995; 105: 549-56.
13) Fluhr JW, Vienne MP, Lauze C, et al. Tolerance
profile of retinol, retinaldehyde and retinoic acid
under maximized and long-term clinical conditions.
Dermatology 1999; 199(S1): 57-60.
14) Yamaguchi Y, Nagasawa T, Nakamura N, et al. Successful
treatment of photo-damaged skin of nano-scale atRA
particles using a novel transdermal delivery. J Control
Release 2005; 104: 29-40.
|

