|
Introduction
Although fat tissue has been used as a filler material
for more than 100 years,1,2 there are several problems
to be resolved, including unpredictability and fat
necrosis resulting in infection and calcification.2,3
Autologous fat transfer, however, is almost the only
method of soft tissue augmentation that can be performed
without detectable scarring on either a donor or a
recipient site and without complications associated
with foreign materials. Because fat tissue is more
easily damaged by ischemia compared to other tissues
such as skin and bone, transferring fat tissue as
quickly as possible after harvesting is recommended.
Thus, it is of great interest to investigate how adipose
tissue viability after aspiration changes over time
depending on preservation methods. Clinically, aspirated
fat tissue is usually preserved at room temperature
in a suction bottle, but how aspirated fat in the
suction bottle changes during the postoperative hours
is unknown.
It was recently revealed that adipose tissue is a
remarkable source of multipotent stem cells,4,5 which
can differentiate into adipogenic, chondrogenic, osteogenic,
myogenic, neurogenic, endothelial, and other lineages6.
Adipose-derived stem cells (ASCs) have already been
used in some clinical trials, including treatments
for bone defects7 and rectovaginal fistula,8 and soft
tissue augmentation such as breast enhancement, breast
reconstruction, and facial rejuvenation.9 ASCs may
be clinically used or banked also for other therapeutic
purposes in the near future. In practice, there is
some time lag between liposuction and cell isolation;
it takes a few to several hours for liposuction surgery
depending on the volume and sites to suction, and
a few hours to even a day or two for transportation
from an operation room to a cell processing unit.
To maximize the potentiality of adipose aspirates
as a stem cell source, it is very important to optimize
protocols for their preservation.
Thus, aspirated fat is now valuable as an autologous
filler material and as an abundant stem cell source.
We sought to comprehensively evaluate the influences
of preservation at differential temperatures on the
viability of aspirated fat and ASCs.
Materials and Methods
Human Tissue Sampling
We obtained liposuction aspirates from 17 healthy
female donors undergoing liposuction of the abdomen
or thighs after informed consent using an IRB-approved
protocol. The adipose portion of the liposuction aspirates
was subjected to assays, as described below. Excised
fat obtained from a tummy-tuck patient was also used
for comparison.
Cell processing and culture
Stromal vascular fractions (SVF) were isolated from
the fatty portion of liposuction aspirates as previously
described.10 Briefly, the aspirated fat was washed
with PBS and digested on a shaker at 37oC in PBS containing
0.075% collagenase for 30 min. Mature adipocytes and
connective tissues were separated from pellets by
centrifugation (800 ×g, 10 min). The pellets were
resuspended and filtered with a 100-μm mesh (Millipore,
MA, USA). Freshly isolated SVF was plated (30,000
cells/cm2) on gelatin-coated dishes and cultured at
37oC in an atmosphere of 5% CO2 in humid air. The
culture medium was M-199 containing 10% FBS, 100 IU
penicillin, 100 mg/mL streptomycin, 5 μg/mL heparin,
and 2 ng/mL acidic FGF. Medium was replaced every
third day. After 7 days, adherent cells were trypsinized
and counted with a cell counter (NucleoCounterTM,
ChemoMetec, Allerod, Denmark).
Flow cytometry analysis
Adherent ASCs were examined for surface marker expression
using flow cytometry after 1 week of culture. The
following monoclonal antibodies were used: CD29-PE,
CD31-PE, CD34-PE, CD45-PE, CD90-PE, CD133-PE, CD144-PE,
HLA-A,B,C-PE, Tie-2-PE (BD Biosciences, San Diego,
CA, USA), CD105-PE (Serotec, Oxford, UK), and Flk-1-PE
(Techne, NJ, USA). Cells were incubated with the directly
conjugated monoclonal antibodies in PBS containing
0.5% bovine serum albumin (BSA) for 30 min at 4oC,
then washed with PBS containing 0.2% BSA and diluted
in PBS containing 0.1% BSA. Flow cytometric analyses
were performed using an LSR2R (Becton Dickinson, San
Jose, CA, USA).
Quantitative analysis of damaged
adipocytes in aspirated fat
To assess damaged adipocytes in aspirated fat, we
measured the ratios of oil and fat volumes after centrifugation,
as follows. The fatty portion of liposuction aspirates
was divided into 20 tubes (15-mL conical tubes) and
preserved at room temperature. After preservation
for 1, 2, 4, and 24 hours, five of the tubes were
centrifuged at 2330 ×g for 5 min to separate the oil,
fat, and fluid into distinct layers from top to bottom
(Fig. 1). The oil ratio was calculated as follows:
oil ratio = (oil volume)/[(oil volume) + (fat volume)].
Data were collected from lipoaspirates obtained from
six patients.
Scanning electron microscope study
After preservation at room temperature or 4oC, aspirated
fat was fixed with 2% paraformaldehyde and 2.5% glutaraldehyde
in 0.2 M cacodylate buffer for a week at room temperature,
and then fixed in 1% osmium tetroxide. After dehydration,
samples were dried with a super critical point CO2
dryer (HCP-2, Hitachi, Tokyo, Japan), sputter-coated
with Pt-Pd, and examined with a scanning electron
microscope (SEM) (S3500N, Hitachi).
Statistical analysis
Results were expressed as mean ± standard error (S.E.).
Paired or unpaired t-tests were used to compare each
parameter.
Results
Morphology of adipocytes in aspirated fat
Aspirated fat was preserved at 4oC and fixed for the
SEM study at different time points. Adipocytes from
aspirated fat almost retained their round shape and
showed no significant morphological differences compared
with those of excised fat (Fig. 2a). No remarkable
change in adipocyte morphology was found in aspirated
fat tissues on days 0, 1, and 3 (Fig. 2b-d).
Aspirated fat was preserved for 1, 2, 4, or 24 hours
at room temperature and evaluated by SEM, as well
(Fig. 2e-h). No remarkable difference in adipocyte
morphology was identified.
Degeneration of adipocytes with preservation
time
Because we clinically experience a gradual increase
of oil volume in lipoaspirates, oil ratio (=oil volume/[oil
volume + fat volume]) was used as an index of degeneration
of adipocytes in aspirated fat. Oil ratio increased
over time during preservation at room temperature
(Fig. 3). The oil ratio at 4 hours was greater than
that at 1 hour, and at 24 hours was significantly
greater than ratios at 1 and 2 hours.
ASC yield from aspirated fat preserved
at room or cool temperature
When preserved at room temperature, ASC yield was
maintained up to 4 hours and remarkably decreased
at 24 hours (Fig. 4). On the other hand, after preservation
at 4oC, almost the same number of ASCs was isolated
from aspirated adipose tissue on days 0 and 1 (Fig.
5) The number of isolated ASCs was extensively decreased
in some cases on day 2 and in all cases on day 3.
Statistical significance was seen between days 0 and
3 (P<0.001) and between days 1 and 3 (P<0.001).
Surface marker expression of ASCs
isolated from aspirated fat preserved at a cool temperature
To examine changes in the biological properties of
ASCs based on preservation time at a cool temperature,
surface marker analysis was performed on ASCs isolated
from aspirated fat tissues preserved for 0, 1, 2,
and 3 days at 4oC (Table 1).
ASC yield from cryopreserved aspirated
fat
We also evaluated the possibility of isolating ASCs
from aspirated fat cryopreserved for 30 days (n=3).
ASCs were harvested from the cryopreserved aspirated
fat, but the ASC yield was significantly less than
that obtained from fresh aspirated fat (Fig. 6).
Discussion
The SEM assay showed that no significant morphological
change in adipocytes was found among aspirated fat
tissues preserved either at 4oC for up to 3 days or
at room temperature for up to 24 hours. However, quantitative
analysis by measuring the oil ratio revealed that
preserved adipocytes were partly degenerated and ruptured
over preservation time when stored at room temperature.
Thus, preservation at room temperature resulted in
damage to some adipocytes that may have been located
superficially; however, the remaining adipocytes retained
almost-intact morphology.
In this study, damaged adipocytes were evaluated by
measurement of the oil ratio after centrifugation.
Boschert et al.11 reported that centrifugation at
greater than 100 ×g caused adipose cell destruction;
however, we recently found that centrifugation at
400 ×g increased the oil portion in lipoaspirates
but further centrifugation did not significantly damage
adipocytes or increase oil volume.12 In addition,
our histological examinations of centrifuged aspirated
fat with light and scanning electron microscopes showed
that adipocytes appeared to be intact even after centrifugation
4300 ×g.12 Therefore, we considered that the increased
oil volume after centrifugation (for 5 min at 2330
×g) in the present study was attributable to adipocyte
damage from preservation at room temperature. Thus,
the present result indicated preservation at room
temperature for 4 hours significantly damaged adipocytes
in aspirated fat; thus, lipotransfer should be performed
as quickly as possible after aspiration, especially
when a large volume of aspirated fat is to be transplanted.
Since the reports showing that adipose tissue contains
multipotent stem cells,4,5 aspirated adipose tissue
has been regarded as not only a filler material but
also as an abundant source of stem cells. ASCs reside
in adipose tissue as progenitors of adipocytes, but
it has been suggested that ASCs can differentiate
into vascular endothelial cells,13,14 can release
angiogenetic factors under hypoxic conditions,15 and
can contribute to a higher graft take of transplanted
fat.14,16 In the current study, ASC yield was maintained
up to 4 hours at room temperature, and an ASC yield
similar to that of fresh aspirated fat was obtained
from that preserved at 4oC for 24 hours. This finding
indicates that a one-day delay in isolation of ASCs
from aspirated fat can be appropriate when the tissue
is stored in a refrigerator. Therefore, overnight
cooling transportation of aspirated fat to a specialized
cell processing center for isolation and banking of
ASCs can be regarded as practical, although ASC yield
after 2 or 3 days would be uncertain, even with preservation
at 4 oC.
Isolated ASCs can be frozen, thawed, and cultured
again as well as almost any other cell type. However,
whether aspirated fat tissue can be frozen as an effective
filler material or a source of ASCs has not yet been
established. In this study, we tried to isolate ASCs
from aspirated fat cryopreserved for 30 days as well
as from fresh aspirated fat. The ASC yield from cryopreserved
fat was much lower than that of fresh aspirated fat.
We tried several kinds of freezing conditions (rapid
or slow freezing) and other freezing media (DMEM or
M199 containing 10% DMSO with 1% methylcellulose or
1% trehalose or 1-20% gelatin, or their mixture),
but the ASC yield was not improved (data not shown).
Although there were a number of red blood cells contaminating
cell fractions isolated from fresh aspirated fat,
almost no red blood cells contaminated those from
cryopreserved aspirated fat. Our result with ASC yield
from cryopreserved fat contradicts a recent report17
showing that the ASC yield from cryopreserved lipoaspirates
was about 90% of that from fresh lipoaspirates. The
reported ASC yield from cryopreserved adipose is 3.7
± 1.4 × 105 cells/mL after 2-week culture,17 which
is comparable to our result (6.7 ± 4.7 × 104 cells/mL
after a 1-week culture) because ASCs proliferate 10?100
times in a week depending on culture conditions. However,
the reported ASC yield from fresh adipose (4.1 ± 1.4
× 105 cells/mL after 2-week culture17) was much less
than that in this study (7.9 ± 1.5 × 105 cells/mL
after 1-week culture). It is unknown why reported
ASC yields from fresh adipose differ between the two
studies, but it may the result of different methods
of cell isolation.
In conclusion, we have demonstrated how ASC yield
from aspirated fat changes depending on preservation
conditions and time periods. Preservation for 4 hours
at room temperature significantly damaged adipocytes
but did not significantly alter ASC yield. ASC yield
significantly decreased with preservation for 24 hours
at room temperature but not with preservation at 4oC.
Thus, aspirated fat can be transported to a cell processing
center for cell isolation on the day following harvesting
and for subsequent banking if it is kept at 4oC. ASC
yield from cryopreserved aspirated fat was minimal,
and a further optimization of methodology of freezing
and preservation is needed for practical use of cryopreservation
of aspirated fat intended as an ASC source.
Acknowledgment
We thank Dr. Satoru Fukuda for his assistance in the
histological assay with SEM.
Figure Legends
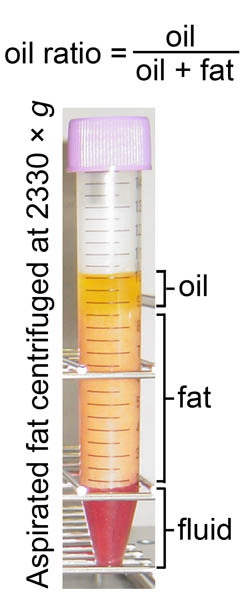
Fig. 1
Analysis of adipocyte damage in lipoaspirates by centrifugation.
After centrifugation, aspirated fat tissue was separated
into distinct layers from top to bottom: the oil,
fat, and fluid layers. Adipocyte damage by preservation
was quantified by calculating the oil ratio in the
volume as follows: oil ratio = (oil volume)/[(oil
volume) + (fat volume)]. Figure 3 shows the results
of the oil ratio calculations.
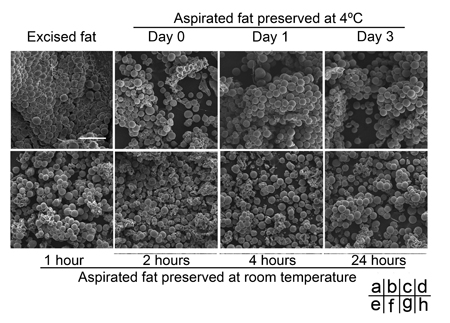
Fig. 2
Comparison with a scanning electron microscope of
human aspirated fat tissues after preservation at
4oC or room temperature.
(a) Excised adipose tissue was fixed immediately after
the operation. (b-h) Aspirated fat tissues preserved
at 4oC were fixed on Day 0 (b), Day 1 (c), or Day
3 (d), while those preserved at room temperature were
fixed at 1 hour (e), 2 hours (f), 4 hours (g), or
24 hours (h) after the operation. Each sample was
treated for evaluation with scanning electron microscopy
(SEM), and representative photos are shown. No significant
morphological changes over time were found by SEM
in aspirated fat, even in samples stored at 4oC for
3 days or at room temperature for 24 hours. Scale
bar: 250 μm.
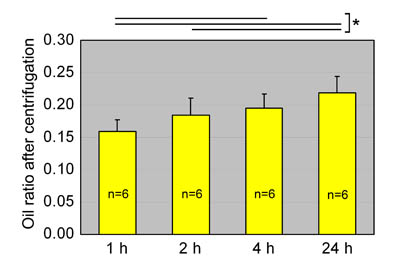
Fig. 3
Oil ratios of aspirated fat preserved at room temperature.
Oil ratios in aspirated fat preserved at room temperature
for 1, 2, 4, or 24 hours are shown. Statistical analysis
was performed using paired t-tests between groups.
The oil volume ratio gradually increased with storage
time, likely because of breakdown of adipocytes. Values
are mean + S.E. *P<0.05.
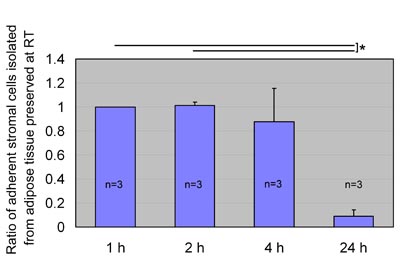
Fig. 4
ASC yield after preservation at room temperature.
We preserved aspirated adipose tissue at room temperature
for 1, 2, 4, or 24 hours and processed for isolation
of ASCs, which were then cultured for 1 week. Ratios
of ASC yield to control (1 hour preservation) were
calculated; data were obtained from three patients,
and statistical analysis was performed using paired
t-tests between groups. ASC yield seemed to be maintained
for up to 4 hours of preservation and remarkably decreased
when preserved for 24 hours at room temperature. Values
are mean + S.E. *P<0.05.
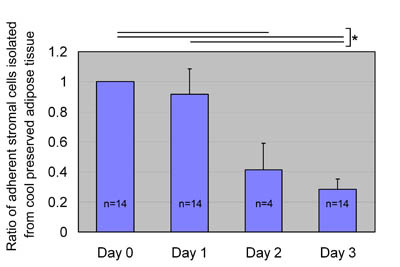
Fig. 5
ASC yields after preservation at 4oC.
We preserved aspirated fat tissues at 4oC for 0, 1,
2, and 3 days and processed them for isolation of
ASCs, which were then cultured for 1 week. Ratios
of ASC yield to control (Day 0: no preservation) were
calculated; data were obtained from 14 patients (data
for Day 2 came from 4 of the 14 patients), and statistical
analysis was performed using unpaired t-tests between
groups. A statistical difference in ASC yield was
not found between days 0 and 1, whereas ASC yield
significantly decreased on days 2 and 3. Values are
mean + S.E. *P<0.05.
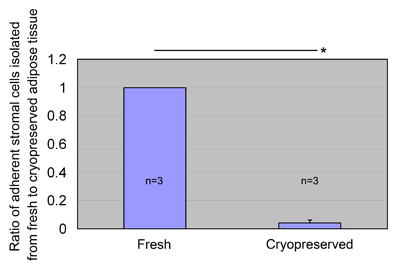
Fig. 6
ASC yields from cryopreserved lipoaspirates.
Fresh aspirated adipose tissue was mixed with an equal
amount of freezing medium, cooled to -80oC in a programmable
freezing system, and stored at -80oC for 1 month.
The cryopreserved adipose tissue was thawed and processed
to isolate ASCs, which were then cultured for 1 week.
The ratio of ASC yield to control (fresh lipoaspirates)
was calculated. Data were obtained from three patients,
and statistical analysis was performed using paired
t-tests between groups. ASCs were isolated from cryopreserved
aspirated fat (6.7 ± 4.7 × 104 cells/mL after 1 week
culture), but the yield was much less than that of
the fresh fat. Values are mean + S.E. *P<0.05.
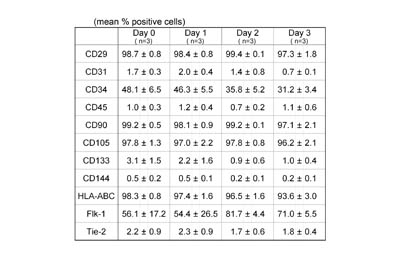
Table 1
Surface marker expression of ASCs isolated from aspirated
fat tissues preserved at 4oC.
ASCs were isolated from aspirated fat preserved at
4oC for 0, 1, 2, and 3 days, and flowcytometric analyses
were performed on ASCs after culture for 1 week. Few
differences in expression profile of principal surface
markers were observed among groups, suggesting that
biological properties of ASCs do not change by preservation
at 4oC for up to 3 days. Values are mean ± S.E.
References
1. Neuber, K. Fettgewebstransplantation. Verh. Dtsch.
Ges. Chir. 1: 66, 1893.
2. Shiffman, M. A., Mirrafati, S. Fat transfer techniques:
the effect of harvest and transfer methods on adipocyte
viability and review of the literature. Dermatol.
Surg. 27: 819, 2001.
3. Ersek, R. A., Chang, P., Salisbury M. A. Lipo layering
of autologous fat: an improved technique with promising
results. Plast. Reconstr. Surg. 101: 820, 1998.
4. Zuk, P. A., Zhu, M., Mizuno, H., et al. Multilineage
cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7: 211, 2001.
5. Zuk, P. A., Zhu, M., Ashjian, P., et al. Human
adipose tissue is a source of multipotent stem cells.
Mol. Biol. Cell. 13: 4279, 2002.
6. Tholpady, S. S., Llull, R., Ogle, R. C., et al.
Adipose tissue: stem cells and beyond. Clin. Plast.
Surg. 33: 55, 2006.
7. Lendeckel, S., Jodicke, A., Christophis, P., et
al. Autologous stem cells (adipose) and fibrin glue
used to treat widespread traumatic calvarial defects:
case report. J. Craniomaxillofac. Surg. 32: 370, 2004.
8. Garcia-Olmo, D., Garcia-Arranz, M., Herreros D.,
et al. A phase I clinical trial of the treatment of
Crohn's fistula by adipose mesenchymal stem cell transplantation.
Dis. Colon Rectum. 48: 1416, 2005.
9. Yoshimura, K., Matsumoto, D., Gonda, K. A clinical
trial of soft tissue augmentation by lipoinjection
with adipose-derived stem cells. Proceedings of 8th
Annual Meeting of Tissue Engineering Society International.
pp125, 2005.
10. Yoshimura, K., Shigeura, T., Matsumoto, D., et
al. Characterization of freshly isolated and cultured
cells derived from the fatty and fluid portions of
liposuction aspirates. J. Cell. Physiol. 208: 64,
2006.
11. Boschert, M. T., Beckert, B. W., Puckett, C. L.,
et al. Analysis of lipocyte viability after liposuction.
Plast. Reconstr. Surg. 109: 761, 2002.
12. Kurita, M., Matsumoto, D., Shigeura, T., et al.
Influences of centrifugation on cells and tissues
in liposuction aspirates: optimized centrifugation
for lipotransfer and cell isolation. Plast. Reconstr.
Surg., in press.
13. Miranville, A., Heeschen, C., Sengenes, C., et
al. Improvement of postnatal neovascularization by
human adipose tissue-derived stem cells.
Circulation. 110: 349, 2004.
14. Matsumoto, D., Sato, K., Gonda, K., et al. Cell-assisted
lipotransfer (CAL): supportive use of human adipose-derived
cells for soft tissue augmentation with lipoinjection.
Tissue Eng., in press.
15. Rehman, J., Traktuev, D., Li, J., et al. Secretion
of angiogenic and antiapoptotic factors by human adipose
stromal cells. Circulation. 109: 1292, 2004.
16. Masuda, T., Furue, M., Matsuda, T. Novel strategy
for soft tissue augmentation based on transplantation
of fragmented omentum and preadipocytes. Tissue Eng.
10: 1672, 2002.
17. Pu, L. L., Cui, X., Fink, B. F., et al. Adipose
aspirates as a source for human processed lipoaspirate
cells after optimal cryopreservation. Plast. Reconstr.
Surg. 117: 1845, 2006. |

