| INTRODUCTION
In Caucasians, the most common complaints about photoaged
skin are fine wrinkles and telangiectasia, but these
complaints are less common in populations with darker
skin, such as Asians. In Asians, hyperpigmentation
is the most common cosmetic complaint, but a standard
strategy for treating hyperpigmented skin lesions
has not been established. We have found that aggressive
use of topical tretinoin (0.1-0.4%) along with hydroquinone
(RA-HQ therapy) can successfully treat various skin
hyperpigmentation conditions in Asians.1-8 Although
the bleaching protocol we routinely use requires two
steps (a bleaching step and a healing step), and severe
but transient adverse skin reactions are possible,
this protocol is quite effective in removing epidermal
pigmentation. The rationale for this protocol is that
tretinoin reduces epidermal melanin by accelerating
epidermal turnover and promoting keratinocyte proliferation,
while hydroquinone acts as a suppresser of melanogenesis
by epidermal melanocytes.5,6,9 It is especially useful
for treating pigmented lesions that are not effectively
treated by lasers, such as melasma, postinflammatory
hyperpigmentation (PIH), and pigmented nipples/areolas.
Unfortunately, this bleaching protocol has no effect
on dermal pigmentation. In addition, hyperkeratotic
lesions are not a good indication for this protocol
because the thickened horny layer prevents penetration
and absorption of the agents. Therefore, in order
to treat every kind of hyperpigmented lesion, we have
to additionally use Q-switched lasers or CO2 lasers
to complement the bleaching protocol. Q-switched ruby
laser (QSR) use can be combined with topical treatment
for synergistically treating skin lesions when both
epidermal and dermal pigmentation are present, as
in acquired dermal melanocytosis (ADM),5,6,8 friction
melanosis, and pigmented cosmetic dermatitis. Depending
on their severity, hyperkeratotic lesions can be treated
primarily with QSR or CO2 laser irradiation.
In order to establish a therapeutic strategy that
addresses a wide range of hyperpigmented conditions,
we used topical bleaching treatment, QSR, CO2 lasers,
or combination treatment to treat patients in our
clinic, and analyzed 59 histological specimens from
49 patients. Clinical diagnosis identified 17 types
of lesions, which included conditions rarely described
as treatment targets. This analysis led us to propose
a new classification system for hyperpigmented conditions
based on histological features. Based on our clinical
experience treating hyperpigmented lesions and our
proposed classification system, we also present a
comprehensive therapeutic strategy for treating pigmented
skin conditions.
METHODS
Fifty-nine biopsies were taken from 49 Japanese female
patients (4 males and 45 females, age ranged from
16 to 60 (ave. = 36.16)) with hyperpigmented skin
lesions after informed consent using an IRB-approved
protocol. The lesions were clinically diagnosed as
follows: ADM (n =12); solar (senile) lentigines (n
= 8); lichen pilaris (n = 4); ripple/reticulate hyperpigmentation
in atopic dermatitis (RHAD) (n = 7); nevus spilus
(cafe au lait macules) (n = 4); pigmented contact
dermatitis (n = 4); pigmented nipple areolar complex
(PNAC) (n = 3); pigmentatio petaloides actinica (PPA)
(n = 4); ephelides (n = 2); friction melanosis (n
= 2); periorbital hyperpigmentation (n = 2); nevus
of Ota (n = 2); postinflammatory hyperpigmentation
resulted from a single inflammatory event (PIH-S)
(n = 1); seborrheic keratosis (n = 1); melasma (ML)
(n = 1); pigmented external genitalia (n = 1); and
erythromelanosis follicularis faciei et colli (EFFC)
(n = 1). In addition, 10 samples were taken from 10
patients at baseline and during RA-HQ therapy; these
lesions included diagnoses of solar lentigines (n
= 1), lichen pilaris (n = 2), nevus spilus (n = 1),
pigmented contact dermatitis (n = 2), PNAC (n = 1),
RHAD (n = 1), ADM (n = 1), and PPA (n = 1).
Biopsied tissue was fixed in 4% buffered neutral formaldehyde
solution and embedded in paraffin for sectioning.
The sections were stained with hematoxylin-eosin and
Fontana-Masson stains. The pathological examination
focused on the existence of hyperkeratosis and the
location (layers) of melanin deposits or melanocytes,
since these two factors determined our therapeutic
strategy. Comparing samples obtained before and during
treatment, morphological alterations due to the RA-HQ
therapy were observed.
Details of the RA-HQ therapy protocol were described
previously.4-6 Briefly, the protocol consisted of
a bleaching phase and a healing phase. We originally
prepared 0.1, 0.2, and 0.4% tretinoin aqueous gels
and an ointment with 5% hydroquinone. For bleaching,
tretinoin was first applied only to the pigmented
area with a cotton tip applicator, and then hydroquinone
was applied to the larger area surrounding the lesion
(the whole face, for example). Initially, the application
was done twice a day, depending on skin reactions
such as erythema and scaling. When sufficient reduction
in pigmentation was obtained, the application of tretinoin
was discontinued. The duration of this phase was 2
to 6 weeks. For healing, hydroquinone alone was applied
twice a day until the erythema disappeared completely;
the duration of this phase was usually 4 weeks.
RESULTS
Biopsies of pigmented lesions
The histological characteristics and diagnoses of
the biopsied samples are summarized in Table 1. Clinical
appearance and histology of representative cases of
each morbidity are shown in Figures 1 and 2, respectively.
Horny layers
In general, dermatoses located on the trunk and extremities
rather than on the face have a physiological tendency
toward hyperkeratosis. In agreement with this, in
pigmented lesions located on the body, i.e. on the
nipples/areola or on the external genitalia, the horny
layer was relatively thicker than for pigmented dermatoses
on the face. Solar lentigines and relatively flat
seborrheic keratoses resembled each other histologically.
Both were characterized by distinctive hyperkeratosis,
which was more obvious in lesions located on areas
other than the face. Note that in solar lentigines,
mild hyperkeratosis was observed even in lesions that
appeared flat. Lichen pilaris and EFFC also showed
mild hyperkeratosis, but the hyperkeratosis was more
distinctive in lichen pilaris. This difference in
the degree of hyperkeratosis was greater than expected.
In EFFC, the epidermis was acanthotic and hyperkeratotic
without parakeratosis, and follicular hyperplasia
with follicular plugging was also observed. No other
dermatoses investigated in this study showed hyperkeratosis.
Epidermal pigmentation
Epidermal pigmentation was enhanced in all the morbidities
in this study except for the nevus of Ota case. The
degree of epidermal melanin pigmentation varied among
the morbidities, and substantial differences were
found between cases.
Dermal pigmentation
Dermal melanosis (melanin incontinence) was observed
in some of the morbidities. Lesions resulting from
chronic or repeated inflammation, such as pigmented
contact dermatitis, friction melanosis, RHAD, and
PPA, showed breakdown of the dermo-epidermal junction
and severe dermal melanosis (deposits of melanophages)
in the upper dermis. Some melanophage deposits in
the upper dermis were also seen in cases of melasma
and solar lentigines, although degenerative changes
of the dermo-epidermal junction were not detected.
ADM and periorbital hyperpigmentation (a subtype of
ADM) had dermal melanocytes with a highly pigmented,
elongated dendritic appearance in the upper dermis,
while dermal melanocytes were scattered throughout
the dermis in nevus of Ota. In several morbidities
such as PIH-S, ephelides, and nevus spilus, dermal
pigmentation, either melanosis or melanocytosis, was
not detected irrespective of the degree of epidermal
pigmentation.
Samples taken during RA-HQ treatment
The histological and clinical manifestations of representative
cases of hyperpigmentation are shown in Figures 3
and 4. In sections obtained during RA-HQ therapy,
substantial epidermal hyperplasia, dramatic reduction
of melanin deposits around the basal layer, and temporal
enhancement of parakeratosis were consistently observed
(Figure 3B and D). These changes were most likely
a result of enhanced basal keratinocyte mitosis and
accelerated turnover of the epidermis. Epidermal pigmentation
was significantly reduced, while dermal melanophages
or melanocytes, if any, appeared unchanged (Figure
4B and D). The clinical observations agreed with the
histological findings. In lesions without dermal pigmentation
involvement, the pigmented color was clinically improved
(Figure 3E), while in lesions with dermal pigmentation,
the color change was usually moderate (Figure 4C)
until laser therapy was also used (Figure 4E).
DISCUSSION
Hyperpigmentation in the epidermis and dermis
Although the degree of pigmentation differed among
both morbidities and cases, the mechanisms underlying
melanin deposits in the epidermis can be understood
as follows. Epidermal melanin is continuously provided
to keratinocytes by melanocytes located in the basal
layer, transported to the stratum corneum by keratinocyte
turnover, and finally removed by stratum corneum sloughing.
In healthy skin, the total amount of epidermal melanin,
the main determinant of skin color, is the result
of the balance between production and discharge of
melanin. Production of epidermal melanin is regulated
by the genetic makeup and gene expression profile
of the melanocytes located in the basal layer, but
production can be enhanced by external factors such
as UV irradiation10 and inflammation. Skin phototype
is determined by the genetic makeup of melanocytes,
which is modified by genetic transformation of melanocytes
in morbidities such as solar lentigines11 and PPA.12
Melanin production can be suppressed by agents such
as hydroquinone that are toxic to melanocytes or that
inhibit melanocytes. On the other hand, the discharge
of epidermal melanin is determined mainly by epidermal
turnover, which can be increased by topical retinoids.
On the contrary, external factors such as topical
corticosteroids and aging can attenuate melanin discharge.
The regulatory mechanism of epidermal pigmentation
(skin color) is a key concept for establishing our
bleaching strategy to epidermal hyperpigmentation;
that is a combined use of tretinoin (a discharge enhancer)
and hydroquinone (a production suppressor).
In morbidities such as ephelides,11 nevus spilus,13
melasma,14,15 lichen pilaris, and EFFC,16,17 melanin
production is pathologically enhanced. In addition
to the underlying genetic factors, PNAC and external
genitalia are also influenced by hormones and topical
inflammation, such as that induced by the friction
caused by clothing.18 In solar lentigines and PPA,
UV irradiation induces genetic alterations in melanocytes,
though other internal and external factors can also
be pathogenetic.10,19 On the other hand, PIH-S is
the result of an external factor: in this condition,
melanogenesis is temporally enhanced due to an inflammatory
event. PIH-S usually involves only epidermal hyperpigmentation
without degenerative changes in the dermo-epidermal
junction, and tends to gradually disappear, but occasionally
it can persist for a long time.
Dermal pigmentation is influenced by another mechanism.
Although melanophages are occasionally present in
the normal dermis of Asians,20 they are mainly observed
in pigmented lesions with a grayish appearance known
as areas of “pigmentary incontinence.”21,22 Histologically,
pigmentary incontinence is caused by melanosome translocation
from the epidermis to the dermis, usually following
dermo-epidermal junction damage. Pigmented contact
dermatitis,23 friction melanosis,24 and RHAD25,26
are caused by repeated inflammatory events, which
induce repeated hypermelanogenesis and degeneration
of the dermo-epidermal junction, and result in scattered
melanophages located in the upper dermis. Our results
confirmed the histological features of these lesions,
and also revealed that some lesions classified as
solar lentigines and melasma also have melanophages
in the upper dermis without apparent dermo-epidermal
damage; the pigmentary incontinence of these lesions
may result from inflammatory events or excessive UV
irradiation encountered after the onset of the lesion.
In acquired dermal melanosis and periorbital hyperpigmentation,
there are ectopic and aberrant melanocytes in the
upper dermis (dermal melanocytosis); these conditions
can be distinguished from the pigmented lesions accompanying
dermal melanosis (pigmented incontinence) described
above.27 Nevus of Ota,28 nevus of Ito,29 and Mongolian
spots29 are known to have aberrant melanocytes throughout
the dermis.
Treatment-oriented histological classification
of hyperpigmented lesions
Based on the features of hyperkeratosis and epidermal
and dermal pigmentation discussed above, we propose
a new classification system for pigmented dermatoses;
diagnosis and histological features of each Category
are summarized in Table 2.
Morbidities with apparent hyperkeratosis are classified
in Category I; our strategy suggests that these lesions
should be treated with CO2 lasers. Based on the location
of the pigmentation, the character of the pigmentation
(melanosis or melanocytosis), and the melanocyte activity
(genetically upregulated or temporally upregulated),
lesions can be placed into Categories II to VII. Category
II and III include only epidermal hyperpigmentation
and Category VII includes only dermal hyperpigmentation,
while Categories IV to VI includes lesions with both
epidermal and dermal hyperpigmentation. Melanocytes
may be temporally upregulated by local inflammatory
events such as dermatitis, scratching, and UV irradiation
(Categories II and IV), while melanocytes have undergone
genetic changes leading to upregulation (Categories
III and V). The epidermal hypermelanosis observed
in lesions in Category VI may be due to stimulatory
effects of melanocytes localized in the upper dermis.4,6
Therapeutic strategy for pigmented lesions
based on their histological characteristics
Based on our classification system for pigmented skin
lesions, we have broadened the indications for our
therapeutic strategy. This therapeutic strategy involves
CO2 laser therapy, QSR laser irradiation, and topical
bleaching treatment (RA-HQ therapy), and is schematically
summarized in Figure 5. RA-HQ treatment accelerates
the removal of accumulated melanosomes in the epidermis
and replaces them with much less pigmented cells.
QSR laser irradiation not only reduces dermal melanosis
and melanocytosis, but also removes the pigmented
and hyperkeratotic epidermis associated with solar
lentigines. The former can also be accomplished using
a Q-switched Alexandrite laser, and the latter can
be accomplished with other lasers, such as the Q-switched
ND:Yag laser. A CO2 laser was used to treat lesions
with excessive hyperkeratosis (Category I lesions)
which could not be effectively treated with a QSR
laser. Hyperkeratotic lesions need to be treated with
lasers: because the thickened horny layer prevents
percutaneous absorption of topical ointment, RA-HQ
therapy does not work.
Lesions in Categories II and III are treated only
with topical RA-HQ therapy, except for solar lentigines
with hyperkeratosis. The treatment usually needs to
be performed repeatedly for melasma.4 PIH-S does not
recur after treatment, but ephelides, lentigo simplex,
and nevus spilus (cafe au lait macules) tend to reappear
within a few months unless topical hydroquinone is
used for post-treatment maintenance. Although the
RA-HQ therapy cannot treat dermal pigmentation and
hyperkeratotic lesions, it can be used as a pre-treatment
for QSR laser irradiation in treating Categories IV
to VI lesions. It can also be used after QSR laser
treatment, because it is quite effective for treating
the PIH frequently seen after QSR laser treatment
of darker-colored skin.
A critical difference between lesions in Category
IV to VI and those in Category VII is the extent of
epidermal pigmentation, which determines the beneficial
and adverse effects of QSR laser on dermal pigmentation.
If the lesion has only dermal melanocytosis (Category
VII), it can be effectively treated with repeated
sessions of QSR laser irradiation alone.30,31 However,
the sole use of Q-switched laser irradiation failed
in the treatment of morbidities with both epidermal
and dermal pigmentation (Categories IV to VI), ,resulting
in high frequency of PIH and/or depigmentation.32-34
We believe that, in these morbidities, epidermal melanin
deposits obstruct laser irradiations to dermal pigmentation
as competing chromophores; laser irradiation thus
induces considerable inflammation in the epidermis
and, consequently, severe PIH results especially in
patients with darker-colored skin.6 To overcome these
problems, we propose that topical bleaching be performed
prior to QSR laser therapy. The pretreatment can be
applied not only to ADM, but also to other skin conditions
with both epidermal and dermal pigmentation, such
as friction melanosis, pigmented contact (cosmetic)
dermatitis, and RHAD (lesions in Categories IV to
VI; Figure 4). For Category VI lesions (dermal melanocytosis),
the combination of RA-HQ treatment and QSR laser must
usually be performed 2 or 3 times, while only one
QSR session is usually needed for treating lesions
in Categories VI and V (dermal melanosis).
CONCLUSONS
We refined a strategy for treating hyperpigmentation
by studying lesion histology and by reviewing our
extensive clinical experience in treating Asian skin.
Here is our proposed standard strategy: first, treat
epidermal pigmentation with RA-HQ, unless the lesion
has hyperkeratosis; second, treat dermal pigmentation
without epidermal hypermelanosis with repeated QSR
laser irradiation; third, treat dermal pigmentation
with substantial epidermal pigmentation with a combined
RA?HQ treatment and QSR irradiation; fourth, repeat
each session as needed. We believe that the classification
system and the histology-based treatment principles
proposed in this study may be helpful for establishing
a standardized treatment algorithm for hyperpigmented
skin lesions, especially in Asians.
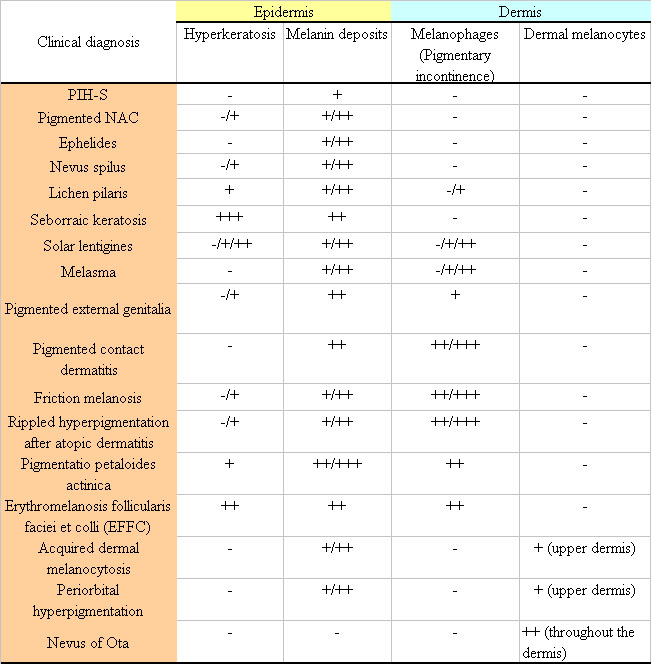
Table 1. A summary of the histological characteristics
of samples stained with Fontana-Masson stain.
Clinical diagnosis Epidermis Dermis
Hyperkeratosis Melanin deposits Melanophages (Pigmentary
incontinence) Dermal melanocytes
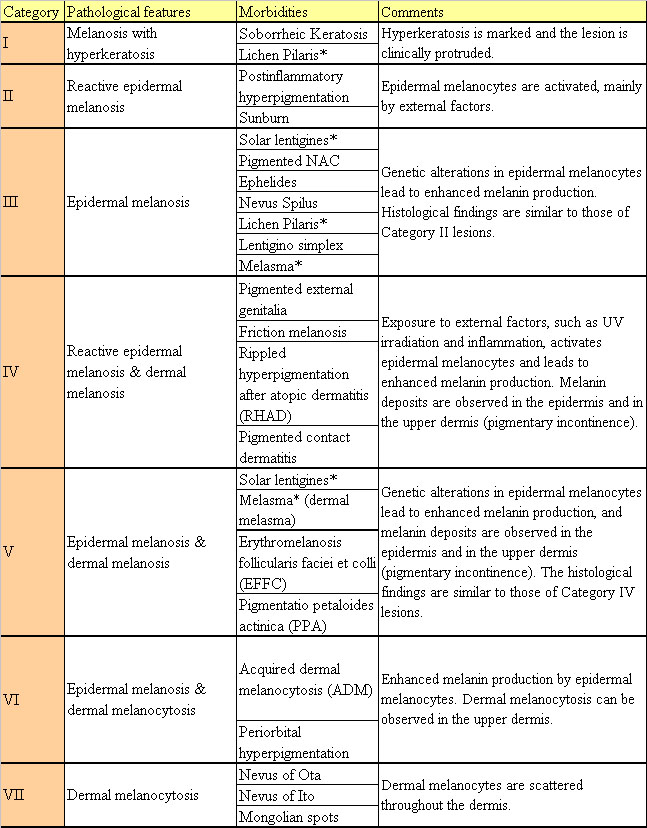
Table 2. Proposed 7-category classification system
for hyperpigmented lesions
* Morbidities classified into two Categories.
Figure legends
b.jpg)
Figure 1. Hyperpigmented lesions in Asians: clinical
manifestations.
(A) Postinflammatory hyperpigmentation resulting from
a single inflammatory event. (B) Pigmented nipple/areola.
(C) Ephelides. (D) Nevus spilus. (E) Lichen pilaris.
(F) Seborrheic keratoses. (G) Solar lentigines. H)
Melasma. (I) Pigmented external genitalia. (J) Pigmented
contact dermatitis. (K) Friction melanosis. (L) Ripple
hyperpigmentation in atopic dermatitis. (M) Pigmentatio
petaloides actinica. (N) Erythromelanosis follicularis
faciei. (O) Acquired dermal melanosis. (P) Periorbital
hyperpigmentation. (Q) Nevus of Ota.
b.jpg)
Figure 2. Hyperpigmented lesions in Asians: histological
findings.
Specimens were obtained from the lesions shown in
Figure 1. Photos A through Q: original magnification
×100. Photos O’, P’, and Q’: corresponding ×200 magnification
photos of specimens O, P, and Q.
b.jpg)
Figure 3. A 26-year-old-woman with pigmented nipple-areola
complex. (A) At baseline, the nipple-areola complex
showed dark brown hyperpigmentation. (B) Histological
examination revealed epidermal pigmentation. (C) Two
weeks after starting bleaching treatment with 0.2%
tretinoin and 5% hydroquinone, the hyperpigmentation
had improved. Some tretinoin-associated erythema was
observed. (D) Histological examination revealed hyperplasia
of the epidermis. Epidermal pigmentation was highly
reduced. (E) Eight weeks after starting treatment
(final result). For 4 weeks, 0.2% tretinoin was used
together with 5% hydroquinone, followed by treatment
with 5% hydroquinone alone for 4 weeks. Pigmentation
improved and no post-inflammatory hyperpigmentation
was observed.
b.jpg)
Figure 4. A 56-year-old woman with pigmented contact
(cosmetic) dermatitis. (A) At baseline, the patient
showed dark brown or dark grey macules distributed
symmetrically on a wide area of the face. (B) Histology
at baseline. The dermo-epidermal junction was severely
damaged and a number of melanosomes were found in
the upper dermis (pigmentary incontinence). (C) At
8 weeks, just after the topical bleaching treatment
with 0.1 and 0.4 % tretinoin and 5% hydroquinone,
the pigmentation was reduced with slight erythema,
but the macules still had a grayish color. (D) Histology
at 8 weeks. Epidermal pigmentation was significantly
improved, while the dermal melanocytosis appeared
not to change at all. (E) Clinical appearance at 20
weeks. QSR irradiation was performed to reduce dermal
melanosis at 8 weeks. Four weeks after the QSR irradiation,
RA-HQ therapy was performed again: 4 weeks of the
bleaching phase and 4 weeks of the healing phase.
b.jpg)
Figure 5. Schematic summary of our therapeutic strategy
for pigmented lesions.
A treatment protocol is suggested for lesions in each
Category (I to VII) except for Category II, which
was divided into three subclasses: solar lentigines,
melasma and others. Solar lentigines frequently have
a thicker horny layer, so that it is initially treated
with a Q-switch laser followed by RA-HQ therapy for
post-laser hyperpigmentation. Melasma sometimes requires
two or three sessions of the RA-HA therapy. Category
IV to VI lesions require combination protocols using
QSR laser and RA-HQ therapy, while Category VII can
be treated with QSR laser treatment alone.
REFERENCES
1. Yoshimura, K, Harii, K, Aoyama, T, et al. A new bleaching
protocol for hyperpigmented skin lesions with a high
concentration of all-trans retinoic acid aqueous gel.
Aesthetic Plast Surg 1999;23:285-91.
2. Yoshimura, K, Harii, K, Aoyama, T, et al. T. Experience
with a strong bleaching treatment for skin hyperpigmentation
in Orientals. Plast Reconstr Surg 2000;105:1097-108.
3. Yoshimura K, Harii K, Masuda Y, et al. Usefulness
of a narrow-band reflectance spectrophotometer in evaluating
effects of depigmenting treatment. Aesthet Plast Surg
2001; 25: 129-33.
4. Yoshimura K, Momosawa A, Watanabe A, et al. Cosmetic
color improvement of the nipple-areola complex by optimal
use of tretinoin and hydroquinone. Dermatol Surg 2002;28:1153-7.
5. Momosawa A, Yoshimura K, Uchida G, et al. Combined
therapy using Q-switched ruby laser and bleaching treatment
with tretinoin and hydroquinone for acquired dermal
melanocytosis. Dermatol Surg 2003;29:1001-7.
6. Yoshimura K, Sato K, Aiba-Kojima E, et al. Repeated
treatment protocols for melasma and acquired dermal
melanocytosis. Dermatol Surg 2006;32:365-71.
7. Sato K, Matsumoto D, Iizuka F, et al. A clinical
trial of topical bleaching treatment with nanoscale
tretinoin particles and hydroquinone for hyperpigmented
skin lesions. Dermatol Surg, in press.
8. Momosawa A, Kurita M, Ozaki M, et al. Combined Therapy
Using Q-Switched Ruby Laser and Bleaching Treatment
with Tretinoin and Hydroquinone for Periorbital Skin
Hyperpigmentation in Asians. Plast Reconstr Surg, in
press.
9. Yoshimura K, Tsukamoto K, Okazaki M, et al. Effects
of all-trans retinoic acid on melanogenesis in pigmented
skin equivalents and monolayer culture of melanocytes.
J Dermatol Sci 2001;27:S68-75.
10. Ortonne JP. Pigmentary changes of the ageing skin.
Br J Dermatol 1990;122:21-8.
11. Bastiaens M, Hoefnagel J, Westendorp R, et al. Solar
lentigines are strongly related to sun exposure in contrast
to ephelides. Pigment Cell Res 2004;17:225-9.
12. Teraki Y, Nishikawa T. Skin diseases described in
Japan 2004. J Dtsch Dermatol Ges 2005;3:9-25.
13. Cohen HJ, Minkin W, Frank SB. Nevus spilus. Arch
Dermatol 1970;102:433-7.
14. Sanchez NP, Pathak MA, Sato S, et al. Melasma: a
clinical, light microscopic, ultrastructural, and immunofluorescence
study. J Am Acad Dermatol 1981;4:698-710.
15. Kang WH, Yoon KH, Lee ES, et al. Melasma: histopathological
characteristics in 56 Korean patients. Br J Dermatol
2002;146:228-37.
16. Mishima Y, Rudner E. Erythromelanosis follicularis
faciei et colli. Dermatologica 1966;132:269-87.
17. Kim MG, Hong SJ, Son SJ, et al. Quantitative histopathologic
findings of erythromelanosis follicularis faciei et
colli. J Cutan Pathol 2001;28:160-4.
18. Daelos ZD. Discussion for Yoshimura K, Momosawa
A, Watanabe A, et al. Cosmetic color improvement of
the nipple-areola complex by optimal use of tretinoin
and hydroquinone. Dermatol Surg 2002;28:1158.
19. Montagna W, Hu F, Carlisle K. A reinvestigation
of solar lentigines. Arch Dermatol 1980;116:1151-4.
20. Ohkuma M. Presence of melanophages in the normal
Japanese skin. Am J Dermatopathol 1991;13:32-7.
21. Nagao S, Iijima S. Light and electron microscopic
study of Riehl's melanosis. Possible mode of its pigmentary
incontinence. J Cutan Pathol 1974;1:165-75.
22. Masu S, Seiji M. Pigmentary incontinence in fixed
drug eruptions. Histologic and electron microscopic
findings. J Am Acad Dermatol 1983;8:525-32.
23. Serrano G, Pujol C, Cuadra J, et al. Riehl's melanosis:
pigmented contact dermatitis caused by fragrances. J
Am Acad Dermatol 1989;21:1057-60.
24. Siragusa M, Ferri R, Cavallari V, Schepis C. Friction
melanosis, friction amyloidosis, macular amyloidosis,
towel melanosis: many names for the same clinical entity.
Eur J Dermatol 2001;11:545-8.
25. Manabe T, Inagaki Y, Nakagawa S, et al. Ripple pigmentation
of the neck in atopic dermatitis. Am J Dermatopathol
1987;9:301-7.
26. Colver GB, Mortimer PS, Millard PR, et al. The 'dirty
neck'--a reticulate pigmentation in atopics. Clin Exp
Dermatol 1987;12:1-4.
27. Hori Y, Kawashima M, Oohara K, Kukita A. Acquired,
bilateral nevus of Ota-like macules. J Am Acad Dermatol
1984;10:961-4.
28. Hirayama T, Suzuki T. A new classification of Ota's
nevus based on histopathological features. Dermatologica
1991;183:169-72
29. Burkhart CG, Gohara A. Dermal melanocyte hamartoma.
A distinctive new form of dermal melanocytosis. Arch
Dermatol 1981;117:102-4.
30 Watanabe S, Takahashi H. Treatment of nevus of Ota
with the Q-switched ruby laser. N Engl J Med 1994;331:1745-50.
31. Chan HH, Alam M, Kono T, Dover JS. Clinical application
of lasers in Asians. Dermatol Surg 2002;28:556-63.
32. Lowe NJ, Wieder JM, Shorr N, et al. Infraorbital
pigmented skin. Preliminary observations of laser therapy.
Dermatol Surg 1995;21:767-70.
33. Kunachak S, Leelaudomlipi P. Q-switched Nd:YAG laser
treatment for acquired bilateral nevus of ota-like maculae:
a long-term follow-up. Lasers Surg Med 2000;26:376-9.
34. Polnikorn N, Tanrattanakorn S, Goldberg DJ. Treatment
of Hori's nevus with the Q-switched Nd:YAG laser. Dermatol
Surg 2000;26:477-80.
|

